Glycogen synthase kinase 3 activity enhances liver inflammation in MASH
- PMID: 38882600
- PMCID: PMC11179260
- DOI: 10.1016/j.jhepr.2024.101073
Glycogen synthase kinase 3 activity enhances liver inflammation in MASH
Abstract
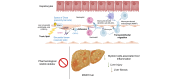
Background & aims: Metabolic dysfunction-associated steatohepatitis (MASH) is characterized by excessive circulating toxic lipids, hepatic steatosis, and liver inflammation. Monocyte adhesion to liver sinusoidal endothelial cells (LSECs) and transendothelial migration (TEM) are crucial in the inflammatory process. Under lipotoxic stress, LSECs develop a proinflammatory phenotype known as endotheliopathy. However, mediators of endotheliopathy remain unclear.
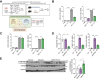
Methods: Primary mouse LSECs isolated from C57BL/6J mice fed chow or MASH-inducing diets rich in fat, fructose, and cholesterol (FFC) were subjected to multi-omics profiling. Mice with established MASH resulting from a choline-deficient high-fat diet (CDHFD) or FFC diet were also treated with two structurally distinct GSK3 inhibitors (LY2090314 and elraglusib [9-ING-41]).
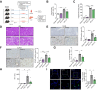
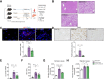
Results: Integrated pathway analysis of the mouse LSEC proteome and transcriptome indicated that leukocyte TEM and focal adhesion were the major pathways altered in MASH. Kinome profiling of the LSEC phosphoproteome identified glycogen synthase kinase (GSK)-3β as the major kinase hub in MASH. GSK3β-activating phosphorylation was increased in primary human LSECs treated with the toxic lipid palmitate and in human MASH. Palmitate upregulated the expression of C-X-C motif chemokine ligand 2, intracellular adhesion molecule 1, and phosphorylated focal adhesion kinase, via a GSK3-dependent mechanism. Congruently, the adhesive and transendothelial migratory capacities of primary human neutrophils and THP-1 monocytes through the LSEC monolayer under lipotoxic stress were reduced by GSK3 inhibition. Treatment with the GSK3 inhibitors LY2090314 and elraglusib ameliorated liver inflammation, injury, and fibrosis in FFC- and CDHFD-fed mice, respectively. Immunophenotyping using cytometry by mass cytometry by time of flight of intrahepatic leukocytes from CDHFD-fed mice treated with elraglusib showed reduced infiltration of proinflammatory monocyte-derived macrophages and monocyte-derived dendritic cells.
Conclusion: GSK3 inhibition attenuates lipotoxicity-induced LSEC endotheliopathy and could serve as a potential therapeutic strategy for treating human MASH.
Impact and implications: LSECs under lipotoxic stress in MASH develop a proinflammatory phenotype known as endotheliopathy, with obscure mediators and functional outcomes. The current study identified GSK3 as the major driver of LSEC endotheliopathy, examined its pathogenic role in myeloid cell-associated liver inflammation, and defined the therapeutic efficacy of pharmacological GSK3 inhibitors in murine MASH. This study provides preclinical data for the future investigation of GSK3 pharmacological inhibitors in human MASH. The results of this study are important to hepatologists, vascular biologists, and investigators studying the mechanisms of inflammatory liver disease and MASH, as well as those interested in drug development.
Keywords: Adhesion; Chemokines; Glycogen synthase kinase 3 (GSK3); Inflammation; Liver fibrosis; Liver sinusoidal endothelial cells (LSEC); Metabolic dysfunction associated steatohepatitis (MASH); Migration; Myeloid cells; Non-alcoholic steatohepatitis (NASH).
© 2024 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures




Similar articles
-
Herbal mixture of Platycodon grandiflorum, Cinnamomum cassia, and Asiasarum sieboldii extracts protects against NASH progression via regulation of hepatic steatosis, inflammation, and apoptosis.Phytomedicine. 2025 Sep;145:157077. doi: 10.1016/j.phymed.2025.157077. Epub 2025 Jul 14. Phytomedicine. 2025. PMID: 40684491
-
Deletion of sphingosine 1-phosphate receptor 1 in myeloid cells reduces hepatic inflammatory macrophages and attenuates MASH.Hepatol Commun. 2025 Feb 3;9(2):e0613. doi: 10.1097/HC9.0000000000000613. eCollection 2025 Feb 1. Hepatol Commun. 2025. PMID: 39899672 Free PMC article.
-
Endothelial c-Maf prevents MASLD-like liver fibrosis by regulating chromatin accessibility to suppress pathogenic microvascular cell subsets.JHEP Rep. 2025 Jun 6;7(9):101475. doi: 10.1016/j.jhepr.2025.101475. eCollection 2025 Sep. JHEP Rep. 2025. PMID: 40810103 Free PMC article.
-
How improvements in US FDA regulatory process and procedures led to the drug approval for first ever treatment of a common liver disease.Acta Pharmacol Sin. 2025 Mar;46(3):515-524. doi: 10.1038/s41401-024-01396-4. Epub 2024 Nov 7. Acta Pharmacol Sin. 2025. PMID: 39511464 Review.
-
Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis.Cochrane Database Syst Rev. 2013 Dec 27;2013(12):CD008623. doi: 10.1002/14651858.CD008623.pub2. Cochrane Database Syst Rev. 2013. PMID: 24374462 Free PMC article.
Cited by
-
Towards Linking Histological Changes to Liver Viscoelasticity: A Hybrid Analytical-Computational Micromechanics Approach.ArXiv [Preprint]. 2025 Jan 2:arXiv:2411.13530v3. ArXiv. 2025. Update in: Phys Med Biol. 2025 Feb 04;70(4). doi: 10.1088/1361-6560/adaad3. PMID: 39606726 Free PMC article. Updated. Preprint.
-
Molecular Landscape and Diagnostic Model of MASH: Transcriptomic, Proteomic, Metabolomic, and Lipidomic Perspectives.Genes (Basel). 2025 Mar 29;16(4):399. doi: 10.3390/genes16040399. Genes (Basel). 2025. PMID: 40282358 Free PMC article. Review.
-
Towards linking histological changes to liver viscoelasticity: a hybrid analytical-computational micromechanics approach.Phys Med Biol. 2025 Feb 4;70(4):10.1088/1361-6560/adaad3. doi: 10.1088/1361-6560/adaad3. Phys Med Biol. 2025. PMID: 39813799 Free PMC article.
-
Metabolic-Dysfunction-Associated Steatotic Liver Disease: Molecular Mechanisms, Clinical Implications, and Emerging Therapeutic Strategies.Int J Mol Sci. 2025 Mar 25;26(7):2959. doi: 10.3390/ijms26072959. Int J Mol Sci. 2025. PMID: 40243565 Free PMC article. Review.
-
The role of liver sinusoidal endothelial cells in metabolic dysfunction-associated steatotic liver diseases and liver cancer: mechanisms and potential therapies.Angiogenesis. 2025 Feb 3;28(2):14. doi: 10.1007/s10456-025-09969-5. Angiogenesis. 2025. PMID: 39899173 Review.
References
-
- Younossi Z.M. Non-alcoholic fatty liver disease – a global public health perspective. J Hepatol. 2019;70:531–544. - PubMed
-
- Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

