Deciphering the enhanced translocation of Pb, Ni and Cd from artificially polluted soil to Chrysopogon zizanioides augmented with Bacillus xiamenensis VITMSJ3
- PMID: 38882641
- PMCID: PMC11178750
- DOI: 10.1007/s13205-024-04001-x
Deciphering the enhanced translocation of Pb, Ni and Cd from artificially polluted soil to Chrysopogon zizanioides augmented with Bacillus xiamenensis VITMSJ3
Abstract
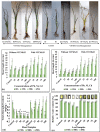
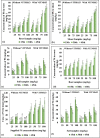
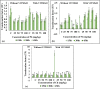
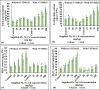
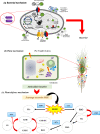
The translocation of heavy metals (HMs) from the rhizosphere to plant systems constitutes a fundamental mechanism governing HM uptake. Microbial augmentation has emerged as a promising strategy to enhance this process. The study investigates the mechanism of enhanced translocation of heavy metals (HMs) from artificially polluted soil to Chrysopogon zizanioides, facilitated by Bacillus xiamenensis VITMSJ3. Pb, Ni, and Cd translocation to the roots and shoots of C. zizanioides was examined, revealing a significant increase of over 15% in HM uptake upon treatment with Bacillus xiamenensis VITMSJ3 (Accession number MT822866). VITMSJ3 exhibited biofilm formation capabilities, attributed to quorum sensing molecule production, and demonstrated resistance to Pb and Ni upto 4000 ppm and Cd upto 450 ppm, respectively. Moreover, VITMSJ3 displayed plant growth-promoting bacterial (PGPB) traits such as, indole-3-acetic acid (IAA), phosphate, ammonia, siderophore, and hydrogen cyanide (HCN) production. Amplification of candidate genes responsible for HM resistance (pbr for Pb, ncc for Ni, cadA for Cd) corroborated the genetic basis of resistance. SEM-EDAX micrographs confirmed HM uptake and translocation along with the presence of VITMSJ3. Enzymatic analysis revealed the synthesis of superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase (GST), peroxidase (POD), and ascorbate peroxidase (APX), implicating their involvement in ROS detoxification. Overall, the study underscores the efficacy of B. xiamenensis VITMSJ3 in enhancing HM translocation, thereby elucidating its potential for phytoremediation applications.
Supplementary information: The online version contains supplementary material available at 10.1007/s13205-024-04001-x.
Keywords: Antioxidant enzymes; Biofilm; Plant growth promoting bacteria; Quorum sensing; Translocation.
© King Abdulaziz City for Science and Technology 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestAuthors declare no conflict of interest regarding the manuscript.
Figures






Similar articles
-
Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils.Plant Physiol Biochem. 2020 Jul;152:90-99. doi: 10.1016/j.plaphy.2020.04.039. Epub 2020 May 3. Plant Physiol Biochem. 2020. PMID: 32408178
-
The analysis of changes in antioxidant enzyme activity and gene expression caused by lead contamination in Azolla caroliniana.Int J Phytoremediation. 2025 Jun 28:1-14. doi: 10.1080/15226514.2025.2521402. Online ahead of print. Int J Phytoremediation. 2025. PMID: 40580003
-
Silicon (Si) foliar treatment modulates Capsicum annuum L. (green chilli) growth and stress responses under cadmium and lead stress.Front Plant Sci. 2025 Jun 13;16:1590148. doi: 10.3389/fpls.2025.1590148. eCollection 2025. Front Plant Sci. 2025. PMID: 40584848 Free PMC article.
-
The role of endophytic bacteria in enhancing plant growth and health for sustainable agriculture.Antonie Van Leeuwenhoek. 2025 Jun 8;118(7):88. doi: 10.1007/s10482-025-02100-0. Antonie Van Leeuwenhoek. 2025. PMID: 40483647 Review.
-
Systematic evaluation of plant metals/metalloids accumulation efficiency: a global synthesis of bioaccumulation and translocation factors.Front Plant Sci. 2025 Jun 5;16:1602951. doi: 10.3389/fpls.2025.1602951. eCollection 2025. Front Plant Sci. 2025. PMID: 40538879 Free PMC article. Review.
Cited by
-
Biodegradation of monocrotophos, cypermethrin & fipronil by Proteus myxofaciens VITVJ1: A plant - microbe based remediation.Heliyon. 2024 Sep 7;10(18):e37384. doi: 10.1016/j.heliyon.2024.e37384. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309857 Free PMC article.
References
-
- Abd El-Rahman AF, Shaheen HA, Abd El-Aziz RM, Ibrahim DSS. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt J Biol Pest Control. 2019 doi: 10.1186/s41938-019-0143-7. - DOI
-
- Abou-Shanab RAI, van Berkum P, Angle JS. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere. 2007;68:360–367. doi: 10.1016/j.chemosphere.2006.12.051. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

