The efficacy and safety of bilateral synchronous transcutaneous auricular vagus nerve stimulation for prolonged disorders of consciousness: a multicenter, double-blind, stratified, randomized controlled trial protocol
- PMID: 38882693
- PMCID: PMC11176550
- DOI: 10.3389/fneur.2024.1418937
The efficacy and safety of bilateral synchronous transcutaneous auricular vagus nerve stimulation for prolonged disorders of consciousness: a multicenter, double-blind, stratified, randomized controlled trial protocol
Abstract
Background: Treatment of disorders of consciousness (DOC) poses a huge challenge for clinical medicine. Transcutaneous auricular vagus nerve stimulation (taVNS) is a non-invasive neuromodulation method, which shows potential in improving recovery of DOC. However, the evidence came from single-center, small-sample randomized controlled trial, which is insufficient to form a conclusion. Thereby, we propose a prospective, multicenter, double-blind, stratified, two-arm randomized controlled trial protocol to investigate the efficacy and safety of bilateral synchronous taVNS for treatment of DOC.
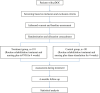
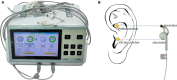
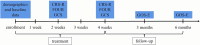
Methods: We aim to recruit 382 patients with prolonged DOC, and divide them into an active stimulation group and a sham stimulation group. The patients in the active stimulation group will receive bilateral synchronous taVNS with a 200 μs pulse width, 20 Hz frequency, and personal adjusted intensity. The sham stimulation group will wear the same stimulator but without current output. Both groups will receive treatment for 30 min per session, twice per day, 6 days per week lasting for 4 weeks. The clinical assessment including Coma Recovery Scale-Revised (CRS-R), Full Outline of Unresponsiveness (FOUR), Glasgow Coma Scale (GCS), and Extended Glasgow Outcome Scale (GOS-E) will be conducted to evaluate its efficacy. Heart rate variability (HRV), blood pressure, and adverse events will be recorded to evaluate its safety.
Discussion: These results will enable us to investigate the efficacy and safety of taVNS for DOC. This protocol will provide multicenter, large-sample, high-quality Class II evidence to support bilateral synchronous taVNS for DOC, and will advance the field of treatment options for DOC.Clinical trial registration:https://www.chictr.org.cn/showproj.html?proj=221851, ChiCTR2400081978.
Keywords: coma recovery scale-revised; disorders of consciousness; heart rate variability; randomized controlled trial; transcutaneous auricular vagus nerve stimulation.
Copyright © 2024 Wang, Yang, Liu, Zhou, Huang, Zou, Feng and Bai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Randomized trial of transcutaneous auricular vagus nerve stimulation on patients with disorders of consciousness: A study protocol.Front Neurol. 2023 Apr 13;14:1116115. doi: 10.3389/fneur.2023.1116115. eCollection 2023. Front Neurol. 2023. PMID: 37122310 Free PMC article.
-
Optimizing the modulation paradigm of transcutaneous auricular vagus nerve stimulation in patients with disorders of consciousness: A prospective exploratory pilot study protocol.Front Neurosci. 2023 Mar 15;17:1145699. doi: 10.3389/fnins.2023.1145699. eCollection 2023. Front Neurosci. 2023. PMID: 37008222 Free PMC article.
-
The efficacy and safety of transcutaneous auricular vagus nerve stimulation for patients with minimally conscious state: a sham-controlled randomized double-blind clinical trial.Front Neurosci. 2023 Dec 14;17:1323079. doi: 10.3389/fnins.2023.1323079. eCollection 2023. Front Neurosci. 2023. PMID: 38156271 Free PMC article.
-
Transcutaneous auricular vagus nerve stimulation in disorders of consciousness: A mini-narrative review.Medicine (Baltimore). 2022 Dec 16;101(50):e31808. doi: 10.1097/MD.0000000000031808. Medicine (Baltimore). 2022. PMID: 36550876 Free PMC article. Review.
-
Current status and prospect of transcutaneous auricular vagus nerve stimulation for disorders of consciousness.Front Neurosci. 2024 Jan 8;17:1274432. doi: 10.3389/fnins.2023.1274432. eCollection 2023. Front Neurosci. 2024. PMID: 38260020 Free PMC article. Review.
Cited by
-
Case report: Monitoring consciousness with fNIRS in a patient with prolonged reduced consciousness following hemorrhagic stroke undergoing adjunct taVNS therapy.Front Neurosci. 2025 Feb 4;19:1519939. doi: 10.3389/fnins.2025.1519939. eCollection 2025. Front Neurosci. 2025. PMID: 39967804 Free PMC article.
References
LinkOut - more resources
Full Text Sources

