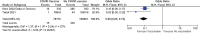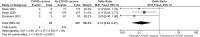Efficacy, Effectiveness, and Safety of COVID-19 Vaccine Compared to Placebo in Preventing COVID-19 Infection among 12-17 Years Old: A Systematic Review
- PMID: 38882914
- PMCID: PMC11168957
- DOI: 10.47895/amp.v58i7.7930
Efficacy, Effectiveness, and Safety of COVID-19 Vaccine Compared to Placebo in Preventing COVID-19 Infection among 12-17 Years Old: A Systematic Review
Abstract
Objectives: The World Health Organization recently revised their recommendations and considered healthy children and adolescents as low priority group for COVID-19 vaccine. This review comprehensively assessed existing clinical evidence on COVID-19 vaccine in 12-17 years old.
Methods: Included in this review were any type of study that investigated the efficacy, immunogenicity, safety, and effectiveness of COVID-19 vaccine on protection against SARS-COV-2 infection in 12-17 years old. Various electronic databases were searched up to March 15, 2023. Studies were screened, data extracted, risk of bias appraised, and certainty of evidence was judged using GRADE. Review Manager 5.4 was used to estimate pooled effects. Difference between the two groups was described as mean difference for continuous variables and as relative risk or odds ratio for categorical variables.
Results: There were six randomized controlled trials and 16 effectiveness studies (8 cohorts and 8 case control). Low certainty evidence showed that BNT162b2 (Pfizer) was effective, immunogenic, and safe in healthy adolescents. There were 15 effectiveness studies on BNT162b2 (Pfizer) in healthy adolescent and one on immunocompromised patients. It was protective against infection with any of the variants, with higher protection against Delta than Omicron. BNT162b2 is protective against hospitalization and emergency and urgent care (high certainty); and critical care and MIS-C (low). Very low certainty evidence noted that BNT 162b2 was also immunogenic in 12-21 years old with rheumatic diseases while on immunomodulatory treatment but with possible increased exacerbation of illness. Low certainty evidence demonstrated that mRNA-1273 (Moderna) was effective, immunogenic, and safe. Low to very low certainty evidence were noted on the safety and immunogenicity of two vector base vaccines (ChAdOx1-19 and Ad5 vector COVID vaccine) and two inactivated vaccines (CoronaVac and BBIBP CorV).
Conclusion: There is presently low certainty evidence on the use of RNA vaccines in 12-17 years old. The recommendation on its use is weak. There is presently insufficient evidence for the use of inactivated and vector-based COVID-19 vaccines. Different countries should consider whether to vaccinate healthy adolescent without comprising the other recommended immunization and health priorities that are crucial for this age group. Other factors including cost-effectiveness of vaccination and disease burden should be accounted.
Keywords: RNA vaccine; inactivated vaccine; vector-based vaccine.
© 2024 Acta Medica Philippina.
Conflict of interest statement
All authors declared no conflicts of interest.
Figures
References
-
- WHO Coronavirus (COVID-19) Dashboard [Internet]. [cited 2023 Apr]. Available from: https://covid19.who.int/?adgroupsurvey={adgroupsurvey}&gclid=CjwKCAjwrpO...
-
- Risk for COVID-19 Infection, Hospitalization, and Death By Age Group [Internet]. [cited 2023 Apr]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-disc...
-
- WHO . SAGE updates COVID-19 vaccination guidance [Internet]. [cited 2023 Mar]. Available from: https://www.who.int/news/item/28-03-2023-sage-updates-covid-19-vaccinati...
LinkOut - more resources
Full Text Sources
Miscellaneous