Development and Safety Trial of the OstreaVent2™ Prototype for Mechanically Ventilated Adult Patients
- PMID: 38882915
- PMCID: PMC11168947
- DOI: 10.47895/amp.v58i7.8329
Development and Safety Trial of the OstreaVent2™ Prototype for Mechanically Ventilated Adult Patients
Abstract
Background: With the surge of COVID-19 infections, there were concerns about shortage of mechanical ventilator in several countries including the Philippines.
Objective: To transform a locally made, low-cost, neonatal ventilator into a volume- and pressure-controlled, adult ventilator and to determine its safe use among ventilated, adult patients at the Philippine General Hospital.
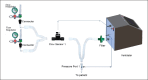
Methods: The modification of the neonatal ventilator (OstreaVent1) to the adult OstreaVent2 was based on the critical need for adult ventilators, in volume or pressure mode, in the Philippines due to the COVID-19 pandemic. The adult ventilator settings were calibrated and tested for two days to check for consistency and tolerance and then submitted to a third party for certification. Once certified, a safety trial of 10 stable adult patients on mechanical ventilator was conducted. The patients were placed on the OstreaVent2 for four hours while ventilator parameters, patient's vital signs, and arterial blood gases were monitored at baseline, during, and after placement on the OstreaVent2. A post-study chest radiograph was also done to rule out pulmonary complications, particularly atelectasis and pneumothorax.
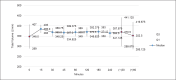
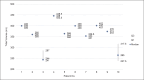
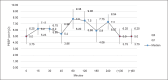
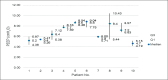
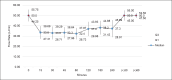
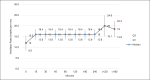
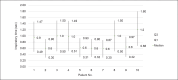
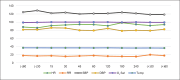
Results: The prototype OstreaVent2 received an FDA Certification for Medical Listing after passing its third-party certification. Ten patients (60% male) recruited in the study had a mean age of 39.1 ± 11.6 years. Half of the patients had a diagnosis of non-COVID-19 pneumonia. During the 4-hour study period, the patients while on the OstreaVent2, had stable ventilator settings and most of the variabilities were within the acceptable tolerances. Vital signs were stable and arterial blood gases were within normal limits. One patient developed alar flaring which was relieved by endotracheal tube suctioning. No patient was withdrawn from the study. One patient who was already transferred out of the ICU subsequently deteriorated and died three days after transfer to the stepdown unit from a non-ventilator related cause.
Conclusion: The new OstreaVent2 is safe to use among adults who need ventilator support. Variabilities in the ventilator's performance were within acceptable tolerances. Clinical and blood gas measurements of the patients were stable while on the ventilator.
Keywords: OstreaVent1; OstreaVent2; adult ventilator.
© 2024 Acta Medica Philippina.
Conflict of interest statement
All authors declared no conflicts of interest.
Figures







References
-
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. .; China Medical Treatment Expert Group for COVID-19 . Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020. Apr 30;382(18):1708-20. doi: 10.1056/NEJMoa2002032. Epub 2020 Feb 28. PMID: 32109013; PMCID: . - DOI - PMC - PubMed
-
- Octaviano AAO, Perez BMB, Benedicto JP. Characteristics and outcomes of COVID-19 patients admitted in the Medical ICU of a tertiary public hospital in the Philippines during the first two months of being a COVID-19 referral center. Acta Med Philipp. 2021. Apr 27;55(2):164-72. doi: 10.47895/amp.v55i2.2671. - DOI
-
- Cabalza D. TIP engineers develop ‘SiglaVent’ ventilator for COVID-19 patients. Philippine Daily Inquirer [Internet]. May 3, 2021. [cited 2020 Apr]. Available from: https://newsinfo.inquirer.net/1426364/tip-engineers-develop-siglavent-ve...
LinkOut - more resources
Full Text Sources
