Targeting nanoplatform synergistic glutathione depletion-enhanced chemodynamic, microwave dynamic, and selective-microwave thermal to treat lung cancer bone metastasis
- PMID: 38883314
- PMCID: PMC11179176
- DOI: 10.1016/j.bioactmat.2024.04.016
Targeting nanoplatform synergistic glutathione depletion-enhanced chemodynamic, microwave dynamic, and selective-microwave thermal to treat lung cancer bone metastasis
Abstract
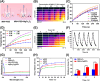
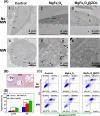
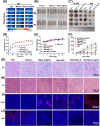
Once bone metastasis occurs in lung cancer, the efficiency of treatment can be greatly reduced. Current mainstream treatments are focused on inhibiting cancer cell growth and preventing bone destruction. Microwave ablation (MWA) has been used to treat bone tumors. However, MWA may damage the surrounding normal tissues. Therefore, it could be beneficial to develop a nanocarrier combined with microwave to treat bone metastasis. Herein, a microwave-responsive nanoplatform (MgFe2O4@ZOL) was constructed. MgFe2O4@ZOL NPs release the cargos of Fe3+, Mg2+ and zoledronic acid (ZOL) in the acidic tumor microenvironment (TME). Fe3+ can deplete intracellular glutathione (GSH) and catalyze H2O2 to generate •OH, resulting in chemodynamic therapy (CDT). In addition, the microwave can significantly enhance the production of reactive oxygen species (ROS), thereby enabling the effective implementation of microwave dynamic therapy (MDT). Moreover, Mg2+ and ZOL promote osteoblast differentiation. In addition, MgFe2O4@ZOL NPs could target and selectively heat tumor tissue and enhance the effect of microwave thermal therapy (MTT). Both in vitro and in vivo experiments revealed that synergistic targeting, GSH depletion-enhanced CDT, MDT, and selective MTT exhibited significant antitumor efficacy and bone repair. This multimodal combination therapy provides a promising strategy for the treatment of bone metastasis in lung cancer patients.
Keywords: Chemodynamic therapy; MgFe2O4@ZOL nanoparticles; Microwave dynamic therapy; Microwave thermal therapy; Targeting.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Facile Synthesis of Fe3O4@Au/PPy-DOX Nanoplatform with Enhanced Glutathione Depletion and Controllable Drug Delivery for Enhanced Cancer Therapeutic Efficacy.Molecules. 2022 Jun 22;27(13):4003. doi: 10.3390/molecules27134003. Molecules. 2022. PMID: 35807249 Free PMC article.
-
Glutathione/pH-responsive copper-based nanoplatform for amplified chemodynamic therapy through synergistic cycling regeneration of reactive oxygen species and dual glutathione depletion.J Colloid Interface Sci. 2023 Dec 15;652(Pt A):329-340. doi: 10.1016/j.jcis.2023.08.043. Epub 2023 Aug 7. J Colloid Interface Sci. 2023. PMID: 37597414
-
Biomimetic nanoplatform with H2O2 homeostasis disruption and oxidative stress amplification for enhanced chemodynamic therapy.Acta Biomater. 2023 May;162:44-56. doi: 10.1016/j.actbio.2023.03.017. Epub 2023 Mar 18. Acta Biomater. 2023. PMID: 36934891
-
Multifunctional CuFe2O4@HA as a GSH-depleting nanoplatform for targeted photothermal/enhanced-chemodynamic synergistic therapy.Colloids Surf B Biointerfaces. 2023 Sep;229:113445. doi: 10.1016/j.colsurfb.2023.113445. Epub 2023 Jul 8. Colloids Surf B Biointerfaces. 2023. PMID: 37441838
-
Nitric oxide-mediated regulation of mitochondrial protective autophagy for enhanced chemodynamic therapy based on mesoporous Mo-doped Cu9S5 nanozymes.Acta Biomater. 2022 Oct 1;151:600-612. doi: 10.1016/j.actbio.2022.08.011. Epub 2022 Aug 9. Acta Biomater. 2022. PMID: 35953045
Cited by
-
Remodeling tumor microenvironment using prodrug nMOFs for synergistic cancer therapy.J Nanobiotechnology. 2025 Feb 19;23(1):123. doi: 10.1186/s12951-025-03202-7. J Nanobiotechnology. 2025. PMID: 39972341 Free PMC article.
-
Recent Advancements in Lung Cancer Metastasis Prevention Based on Nanostrategies.Adv Sci (Weinh). 2025 Jun;12(23):e2409293. doi: 10.1002/advs.202409293. Epub 2025 Mar 26. Adv Sci (Weinh). 2025. PMID: 40135802 Free PMC article. Review.
-
Fucoidan-decorated metal-zoledronic acid nanocomplexes suppress tumor metastasis by inducing ferroptotic cell death and enhancing cancer immunotherapy.J Nanobiotechnology. 2025 Jun 2;23(1):405. doi: 10.1186/s12951-025-03473-0. J Nanobiotechnology. 2025. PMID: 40452005 Free PMC article.
References
-
- Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023;73:17–48. - PubMed
-
- Brody H. Lung cancer. Nature. 2020;587:S7. - PubMed
-
- Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–176. - PubMed
-
- Mundy G.R. Mechanisms of osteolytic bone destruction. Bone. 1991;12(Suppl 1):S1–S6. - PubMed
LinkOut - more resources
Full Text Sources

