Orthogonally woven 3D nanofiber scaffolds promote rapid soft tissue regeneration by enhancing bidirectional cell migration
- PMID: 38883316
- PMCID: PMC11179174
- DOI: 10.1016/j.bioactmat.2024.04.025
Orthogonally woven 3D nanofiber scaffolds promote rapid soft tissue regeneration by enhancing bidirectional cell migration
Abstract
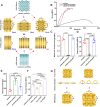
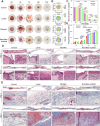
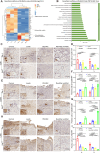
Repairing large-area soft tissue defects caused by traumas is a major surgical challenge. Developing multifunctional scaffolds with suitable scalability and favorable cellular response is crucial for soft tissue regeneration. In this study, we developed an orthogonally woven three-dimensional (3D) nanofiber scaffold combining electrospinning, weaving, and modified gas-foaming technology. The developed orthogonally woven 3D nanofiber scaffold had a modular design and controlled fiber alignment. In vitro, the orthogonally woven 3D nanofiber scaffold exhibited adjustable mechanical properties, good cell compatibility, and easy drug loading. In vivo, for one thing, the implantation of an orthogonally woven 3D nanofiber scaffold in a full abdominal wall defect model demonstrated that extensive granulation tissue formation with enough mechanical strength could promote recovery of abdominal wall defects while reducing intestinal adhesion. Another result of diabetic wound repair experiments suggested that orthogonally woven 3D nanofiber scaffolds had a higher wound healing ratio, granulation tissue formation, collagen deposition, and re-epithelialization. Taken together, this novel orthogonally woven 3D nanofiber scaffold may provide a promising and effective approach for optimal soft tissue regeneration.
Keywords: 3D nanofiber scaffold; Cell migration; Electrospinning; Orthogonal weaving; Tissue regeneration.
© 2024 The Authors.
Conflict of interest statement
All authors declare no conflicts of interest in this work, and all authors have read and agreed to this submission.
Figures





Similar articles
-
Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation.Acta Biomater. 2017 Oct 15;62:102-115. doi: 10.1016/j.actbio.2017.08.043. Epub 2017 Aug 30. Acta Biomater. 2017. PMID: 28864251 Free PMC article.
-
A radial 3D polycaprolactone nanofiber scaffold modified by biomineralization and silk fibroin coating promote bone regeneration in vivo.Int J Biol Macromol. 2021 Mar 1;172:19-29. doi: 10.1016/j.ijbiomac.2021.01.036. Epub 2021 Jan 11. Int J Biol Macromol. 2021. PMID: 33444651
-
Constructing high-strength nano-micro fibrous woven scaffolds with native-like anisotropic structure and immunoregulatory function for tendon repair and regeneration.Biofabrication. 2023 Jan 23;15(2). doi: 10.1088/1758-5090/acb106. Biofabrication. 2023. PMID: 36608336
-
Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation.Acta Biomater. 2019 Sep 15;96:175-187. doi: 10.1016/j.actbio.2019.06.035. Epub 2019 Jun 29. Acta Biomater. 2019. PMID: 31260823
-
3D Electrospun Nanofiber-Based Scaffolds: From Preparations and Properties to Tissue Regeneration Applications.Stem Cells Int. 2021 Jun 17;2021:8790143. doi: 10.1155/2021/8790143. eCollection 2021. Stem Cells Int. 2021. PMID: 34221024 Free PMC article. Review.
References
-
- Wong R., Geyer S., Weninger W., Guimberteau J.-C., Wong J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016;25(2):92–98. - PubMed
-
- Smith D.R., Caban-Rivera D.A., McGarry M.D.J., Williams L.T., McIlvain G., Okamoto R.J., Van Houten E.E.W., Bayly P.V., Paulsen K.D., Johnson C.L. Anisotropic mechanical properties in the healthy human brain estimated with multi-excitation transversely isotropic MR elastography. Brain Multiphysics. 2022;3 - PMC - PubMed
-
- Liu Z., Zhang Z., Ritchie R.O. Structural orientation and anisotropy in biological materials: functional designs and mechanics. Adv. Funct. Mater. 2020;30(10)
-
- Astruc L., De Meulaere M., Witz J.F., Nováček V., Turquier F., Hoc T., Brieu M. Characterization of the anisotropic mechanical behavior of human abdominal wall connective tissues. J. Mech. Behav. Biomed. Mater. 2018;82:45–50. - PubMed
LinkOut - more resources
Full Text Sources

