E3 ligase FBXO22 is not significant for spermatogenesis and male fertility in mice
- PMID: 38883371
- PMCID: PMC11170574
- DOI: 10.62347/STDA4237
E3 ligase FBXO22 is not significant for spermatogenesis and male fertility in mice
Abstract
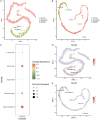
Background: F-box-only protein 22 (FBXO22), an important substrate receptor of the SKP1-Cullin-F-box (SCF) ubiquitin ligases, has been reported to be involved in many biological processes, including tumorigenesis, neurological disorders, cellular senescence, and DNA damage. However, the specific role of FBXO22 during spermatogenesis is poorly understood.
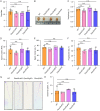
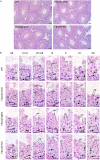
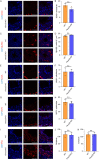
Methods: We produced Fbxo22 conditional knockout (cKO) and global knockout (KO) mice and assessed their sperm masurements using a computer-assisted sperm analysis (CASA) system. Additionally, we conducted histologic staining and immunostaining to examine the impact of Fbxo22 loss on spermatogenesis.
Results: Our results revealed that there were no notable differences in semen quality, fertility test results, or histologic findings in Fbxo22-KO and Fbxo22-cKO mice compared to the control group.
Conclusions: Our study demonstrated that Fbxo22 is not significant for spermatogenesis or male fertility in mice. These findings will help researchers avoid redundant efforts and serve as a foundational resource for genetic studies on human fertility.
Keywords: Fbxo22; knockout; male fertility; spermatogenesis.
AJTR Copyright © 2024.
Conflict of interest statement
None.
Figures


Similar articles
-
Testis-enriched Spsb1 is not required for spermatogenesis and fertility in mice.Am J Transl Res. 2025 Mar 15;17(3):1824-1833. doi: 10.62347/JFJX7128. eCollection 2025. Am J Transl Res. 2025. PMID: 40226024 Free PMC article.
-
FBXO22, an epigenetic multiplayer coordinating senescence, hormone signaling, and metastasis.Cancer Sci. 2020 Aug;111(8):2718-2725. doi: 10.1111/cas.14534. Epub 2020 Jul 17. Cancer Sci. 2020. PMID: 32536008 Free PMC article. Review.
-
Fbxw17 is dispensable for viability and fertility in mice.Mol Biol Rep. 2022 Aug;49(8):7287-7295. doi: 10.1007/s11033-022-07512-z. Epub 2022 May 19. Mol Biol Rep. 2022. PMID: 35585383
-
SCF(Fbxo22)-KDM4A targets methylated p53 for degradation and regulates senescence.Nat Commun. 2016 Feb 12;7:10574. doi: 10.1038/ncomms10574. Nat Commun. 2016. PMID: 26868148 Free PMC article.
-
Genetically engineered mouse models for functional studies of SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases.Cell Res. 2013 May;23(5):599-619. doi: 10.1038/cr.2013.44. Epub 2013 Mar 26. Cell Res. 2013. PMID: 23528706 Free PMC article. Review.
Cited by
-
Testis-enriched Socs7 is not essential for spermatogenesis and male fertility in mice.Am J Transl Res. 2025 Mar 15;17(3):1780-1791. doi: 10.62347/VIVI6495. eCollection 2025. Am J Transl Res. 2025. PMID: 40226015 Free PMC article.
-
DCUN1D2 is insignificant for spermatogenesis and male fertility in mice.Am J Transl Res. 2025 Jul 25;17(7):5614-5624. doi: 10.62347/CLIG5523. eCollection 2025. Am J Transl Res. 2025. PMID: 40821035 Free PMC article.
-
Spsb3 is not essential for spermatogenesis and male fertility in mice.Am J Transl Res. 2025 Mar 15;17(3):1814-1823. doi: 10.62347/TVPV4242. eCollection 2025. Am J Transl Res. 2025. PMID: 40225996 Free PMC article.
-
Testis-enriched Spsb1 is not required for spermatogenesis and fertility in mice.Am J Transl Res. 2025 Mar 15;17(3):1824-1833. doi: 10.62347/JFJX7128. eCollection 2025. Am J Transl Res. 2025. PMID: 40226024 Free PMC article.
-
Dcun1d3 is dispensable for spermatogenesis and male fertility in mice.Am J Clin Exp Immunol. 2025 Jun 15;14(3):127-137. doi: 10.62347/BZPE6333. eCollection 2025. Am J Clin Exp Immunol. 2025. PMID: 40689315 Free PMC article.
References
-
- Bose R, Manku G, Culty M, Wing SS. Ubiquitin-proteasome system in spermatogenesis. Adv Exp Med Biol. 2014;759:181–213. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
