Cepharanthine inhibits migration, invasion, and EMT of bladder cancer cells by activating the Rap1 signaling pathway in vitro
- PMID: 38883391
- PMCID: PMC11170605
- DOI: 10.62347/WDFF7432
Cepharanthine inhibits migration, invasion, and EMT of bladder cancer cells by activating the Rap1 signaling pathway in vitro
Abstract
Background: Cepharanthine, a bioactive constituent of Stephania japonica (Thunb.) Miers, is known for its potent anti-tumor properties. Nevertheless, the precise impact of this substance on bladder cancer remains poorly comprehended. The aim of this study was to demonstrate the effect and mechanism of cepharanthine on the metastasis of human bladder cancer cells.
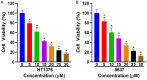
Methods: The application of network pharmacology was utilized to ascertain the possible targets and signaling pathways of cepharanthine in the treatment of bladder cancer. The antiproliferative effects of cepharanthine were evaluated using Cell Counting Kit-8 and colony formation assays. The migration and invasion capabilities were assessed using Transwell assays and wound healing experiments. Proteins related to the Rap1 signaling pathway, cellular migration, cellular invasion, and Epithelial-Mesenchymal Transition (EMT) were quantified by western blotting.
Results: Through database screening, 313 cepharanthine-acting targets, 277 candidate disease targets in bladder cancer, 22 intersecting targets, and 12 core targets were confirmed. The involvement of the Rap1 signaling system was revealed by the Kyoto Encyclopedia of Genes and Genomes' pathway enrichment study. Cepharanthine was shown to decrease bladder cancer cell proliferation, migration, and invasion in vitro. Cepharanthine activated the Rap1 signaling pathway by upregulating Epac1 and downregulating E-cadherin and C3G protein expression, leading to increased expression of Rap1 GTP protein and decreased expression of protein kinase D1 and integrin α5. Rap1 signalling pathway activation resulted in the downregulation of migration and invasion-related proteins, matrix metallopeptidase MMP2, MMP9, as well as EMT-related proteins, N-cadherin and Snail, without affecting vimentin expression.
Conclusion: Cepharanthine inhibits migration, invasion, and EMT of bladder cancer cells by activating the Rap1 signalling pathway. The results offer helpful insights regarding the possible therapeutic use of cepharanthine for treating bladder cancer.
Keywords: Cepharanthine; Rap1 signaling pathway; bladder cancer; cell invasion; cell migration; network pharmacology.
AJTR Copyright © 2024.
Conflict of interest statement
None.
Figures








Similar articles
-
TEAD4 functions as a prognostic biomarker and triggers EMT via PI3K/AKT pathway in bladder cancer.J Exp Clin Cancer Res. 2022 May 17;41(1):175. doi: 10.1186/s13046-022-02377-3. J Exp Clin Cancer Res. 2022. PMID: 35581606 Free PMC article.
-
Melittin Inhibits Ovarian Cancer Cell Growth by Downregulating MMP9 Expression via the JAK2-STAT3 Signaling Pathway.Curr Med Chem. 2025 Apr 29. doi: 10.2174/0109298673355946250403071132. Online ahead of print. Curr Med Chem. 2025. PMID: 40304331
-
CAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transition.Urol Oncol. 2014 Aug;32(6):855-63. doi: 10.1016/j.urolonc.2014.01.005. Epub 2014 Jun 23. Urol Oncol. 2014. PMID: 24968949
-
MicroRNA-124-3p suppresses cell migration and invasion by targeting ITGA3 signaling in bladder cancer.Cancer Biomark. 2019;24(2):159-172. doi: 10.3233/CBM-182000. Cancer Biomark. 2019. PMID: 30614803
-
[SHOX2 promotes migration, invasion and stemness of bladder cancer cells in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Jul 20;41(7):995-1001. doi: 10.12122/j.issn.1673-4254.2021.07.05. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34308848 Free PMC article. Chinese.
Cited by
-
Prognostic and immunological implications of heterogeneous cell death patterns in prostate cancer.Cancer Cell Int. 2024 Aug 24;24(1):297. doi: 10.1186/s12935-024-03462-7. Cancer Cell Int. 2024. PMID: 39182081 Free PMC article.
-
Integrating molecular docking and network pharmacology to reveal the molecular mechanisms of Anemarrhena asphodeloides in the treatment of non-small cell lung cancer.Discov Oncol. 2025 Jul 24;16(1):1399. doi: 10.1007/s12672-025-03178-8. Discov Oncol. 2025. PMID: 40702358 Free PMC article.
References
-
- Lobo N, Afferi L, Moschini M, Mostafid H, Porten S, Psutka SP, Gupta S, Smith AB, Williams SB, Lotan Y. Epidemiology, screening, and prevention of bladder cancer. Eur Urol Oncol. 2022;5:628–39. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020;324:1980–91. - PubMed
-
- Symeonidis EN, Lo KL, Chui KL, Vakalopoulos I, Sountoulides P. En bloc resection of bladder tumors: challenges and unmet needs in 2022. Future Oncol. 2022;18:2545–58. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
