Extracellular electrophysiology on clonal human β-cell spheroids
- PMID: 38883608
- PMCID: PMC11176477
- DOI: 10.3389/fendo.2024.1402880
Extracellular electrophysiology on clonal human β-cell spheroids
Abstract
Background: Pancreatic islets are important in nutrient homeostasis and improved cellular models of clonal origin may very useful especially in view of relatively scarce primary material. Close 3D contact and coupling between β-cells are a hallmark of physiological function improving signal/noise ratios. Extracellular electrophysiology using micro-electrode arrays (MEA) is technically far more accessible than single cell patch clamp, enables dynamic monitoring of electrical activity in 3D organoids and recorded multicellular slow potentials (SP) provide unbiased insight in cell-cell coupling.
Objective: We have therefore asked whether 3D spheroids enhance clonal β-cell function such as electrical activity and hormone secretion using human EndoC-βH1, EndoC-βH5 and rodent INS-1 832/13 cells.
Methods: Spheroids were formed either by hanging drop or proprietary devices. Extracellular electrophysiology was conducted using multi-electrode arrays with appropriate signal extraction and hormone secretion measured by ELISA.
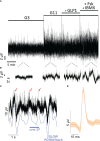
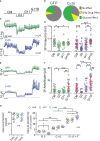
Results: EndoC-βH1 spheroids exhibited increased signals in terms of SP frequency and especially amplitude as compared to monolayers and even single cell action potentials (AP) were quantifiable. Enhanced electrical signature in spheroids was accompanied by an increase in the glucose stimulated insulin secretion index. EndoC-βH5 monolayers and spheroids gave electrophysiological profiles similar to EndoC-βH1, except for a higher electrical activity at 3 mM glucose, and exhibited moreover a biphasic profile. Again, physiological concentrations of GLP-1 increased AP frequency. Spheroids also exhibited a higher secretion index. INS-1 cells did not form stable spheroids, but overexpression of connexin 36, required for cell-cell coupling, increased glucose responsiveness, dampened basal activity and consequently augmented the stimulation index.
Conclusion: In conclusion, spheroid formation enhances physiological function of the human clonal β-cell lines and these models may provide surrogates for primary islets in extracellular electrophysiology.
Keywords: EndoC-βH1; EndoC-βH5; INS-1 cells; extracellular electrophysiology; insulin; islets; microelectrode array; spheroids.
Copyright © 2024 Puginier, Leal-Fischer, Gaitan, Lallouet, Scotti, Raoux and Lang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Characterization of stimulus-secretion coupling in the human pancreatic EndoC-βH1 beta cell line.PLoS One. 2015 Mar 24;10(3):e0120879. doi: 10.1371/journal.pone.0120879. eCollection 2015. PLoS One. 2015. PMID: 25803449 Free PMC article.
-
Renewable Human Cell Model for Type 1 Diabetes Research: EndoC-βH5/HUVEC Coculture Spheroids.J Diabetes Res. 2023 Dec 21;2023:6610007. doi: 10.1155/2023/6610007. eCollection 2023. J Diabetes Res. 2023. PMID: 38162632 Free PMC article.
-
Exploring the functional, protective, and transcriptomic effects of GIP on cytokine-exposed human pancreatic islets and EndoC-βH5 cells.Mol Cell Endocrinol. 2025 May 15;602:112522. doi: 10.1016/j.mce.2025.112522. Epub 2025 Mar 21. Mol Cell Endocrinol. 2025. PMID: 40122442
-
Regulation of insulin secretion in human pancreatic islets.Annu Rev Physiol. 2013;75:155-79. doi: 10.1146/annurev-physiol-030212-183754. Epub 2012 Sep 4. Annu Rev Physiol. 2013. PMID: 22974438 Review.
-
Pancreatic Thyrotropin Releasing Hormone and Mechanism of Insulin Secretion.Cell Physiol Biochem. 2018;50(1):378-384. doi: 10.1159/000494013. Epub 2018 Oct 4. Cell Physiol Biochem. 2018. PMID: 30286449 Review.
Cited by
-
Stimulus-Secretion Coupling Mechanisms of Glucose-Induced Insulin Secretion: Biochemical Discrepancies Among the Canonical, ADP Privation, and GABA-Shunt Models.Int J Mol Sci. 2025 Mar 24;26(7):2947. doi: 10.3390/ijms26072947. Int J Mol Sci. 2025. PMID: 40243540 Free PMC article. Review.
-
Scaffold-free endocrine tissue engineering: role of islet organization and implications in type 1 diabetes.BMC Endocr Disord. 2025 Apr 21;25(1):107. doi: 10.1186/s12902-025-01919-y. BMC Endocr Disord. 2025. PMID: 40259265 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

