Roadmap on Label-Free Super-Resolution Imaging
- PMID: 38883699
- PMCID: PMC11178318
- DOI: 10.1002/lpor.202200029
Roadmap on Label-Free Super-Resolution Imaging
Abstract
Label-free super-resolution (LFSR) imaging relies on light-scattering processes in nanoscale objects without a need for fluorescent (FL) staining required in super-resolved FL microscopy. The objectives of this Roadmap are to present a comprehensive vision of the developments, the state-of-the-art in this field, and to discuss the resolution boundaries and hurdles which need to be overcome to break the classical diffraction limit of the LFSR imaging. The scope of this Roadmap spans from the advanced interference detection techniques, where the diffraction-limited lateral resolution is combined with unsurpassed axial and temporal resolution, to techniques with true lateral super-resolution capability which are based on understanding resolution as an information science problem, on using novel structured illumination, near-field scanning, and nonlinear optics approaches, and on designing superlenses based on nanoplasmonics, metamaterials, transformation optics, and microsphere-assisted approaches. To this end, this Roadmap brings under the same umbrella researchers from the physics and biomedical optics communities in which such studies have often been developing separately. The ultimate intent of this paper is to create a vision for the current and future developments of LFSR imaging based on its physical mechanisms and to create a great opening for the series of articles in this field.
Keywords: Raman microscopy; absorption; artificial intelligence; biomedical imaging; confocal microscopy; deep learning; diffraction; diffraction limit; focusing; high-resolution imaging; holography; interference; label-free imaging; metamaterials; microlens design; microresonators; microspheres; nanoplasmonics; near-field imaging; optical microscopy; photonic crystals; polarization; propagation; reflectivity; scattering; solid immersion lens; spectroscopy; structured illumination; super-resolution; superlens; tomography; transformation optics.
Figures













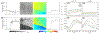




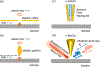

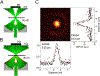
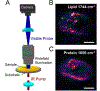

















References
-
- Abbe E, “Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung,” Archiv für mikroskopische AnatomieArchiv für mikroskopische Anatomie Entwichlungsmech 9, 413–468 (1873).
-
- von Helmholtz H, “The theoretical limits of resolving power in the microscope,” Poggendorff Ann. Jubelbd, 557 (1874).
-
- Rayleigh Lord, “On the manufacture and theory of diffraction-gratings,” Philos. Mag 47, 81–93, 193–205 (1874).
-
- Hell SW et al., “The 2015 super-resolution microscopy roadmap,” J. Phys. D: Appl. Phys 48, 443001 (2015).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
