This is a preprint.
Complement-dependent virion lysis mediated by dengue-Zika virus cross-reactive antibodies correlates with protection from severe dengue disease
- PMID: 38883768
- PMCID: PMC11177908
- DOI: 10.1101/2024.06.03.24308395
Complement-dependent virion lysis mediated by dengue-Zika virus cross-reactive antibodies correlates with protection from severe dengue disease
Update in
-
Anti-dengue virus antibodies that elicit complement-mediated lysis of Zika virion correlate with protection from severe dengue disease.Cell Rep. 2025 May 27;44(5):115613. doi: 10.1016/j.celrep.2025.115613. Epub 2025 May 5. Cell Rep. 2025. PMID: 40333188
Abstract
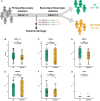
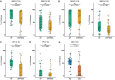
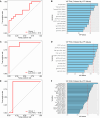
Primary infection with one of four dengue virus serotypes (DENV1-4) may generate antibodies that protect or enhance subsequent secondary heterotypic infections. However, the characteristics of heterotypic cross-reactive antibodies associated with protection from symptomatic infection and severe disease are not well-defined. We selected plasma samples collected before a secondary DENV heterotypic infection that was classified either as dengue fever (DF, n = 31) or dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS, n = 33) from our longstanding pediatric cohort in Nicaragua. We screened various antibody properties to determine the features correlated with protection from DHF/DSS. Protection was associated with high levels of binding of various antibody isotypes, IgG subclasses and effector functions, including antibody-dependent complement deposition, ADCD. Although the samples were derived from DENV-exposed, Zika virus (ZIKV)-naïve individuals, the protective ADCD association was stronger when assays were conducted with recombinant ZIKV antigens. Further, we showed that a complement-mediated virion lysis (virolysis) assay conducted with ZIKV virions was strongly associated with protection, a finding reproduced in an independent sample set collected prior to secondary heterotypic inapparent versus symptomatic DENV infection. Virolysis was the main antibody feature correlated with protection from DHF/DSS and severe symptoms, such as thrombocytopenia, hemorrhagic manifestations, and plasma leakage. Hence, anti-DENV antibodies that cross-react with ZIKV, target virion-associated epitopes, and mediate complement-dependent virolysis are correlated with protection from secondary symptomatic DENV infection and DHF/DSS. These findings may support the rational design and evaluation of dengue vaccines and development of therapeutics.
Conflict of interest statement
Competing interests EH laboratory received research funds from Takeda Vaccines Inc. to test samples from vaccine recipients. EH served on one-time advisory boards for Merck and Takeda. The other authors declare no competing interests.
Figures

References
-
- Postler T. S., Beer M., Blitvich B. J., Bukh J., De Lamballerie X., Drexler J. F., Imrie A., Kapoor A., Karganova G. G., Lemey P., Lohmann V., Simmonds P., Smith D. B., Stapleton J. T., Kuhn J. H., Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol. 168, 224, s00705–023-05835–1 (2023). - PubMed
-
- Cattarino L., Rodriguez-Barraquer I., Imai N., Cummings D. A. T., Ferguson N. M., Mapping global variation in dengue transmission intensity. Sci. Transl. Med. 12, eaax4144 (2020). - PubMed
-
- World Health Organization, Ed., Dengue haemorrhagic fever: diagnosis, treatment, prevention and control (Geneva, 2. ed., 1997).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
