This is a preprint.
Effectiveness of Updated 2023-2024 (Monovalent XBB.1.5) COVID-19 Vaccination Against SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 Lineage Hospitalization and a Comparison of Clinical Severity - IVY Network, 26 Hospitals, October 18, 2023-March 9, 2024
- PMID: 38883802
- PMCID: PMC11177903
- DOI: 10.1101/2024.06.04.24308470
Effectiveness of Updated 2023-2024 (Monovalent XBB.1.5) COVID-19 Vaccination Against SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 Lineage Hospitalization and a Comparison of Clinical Severity - IVY Network, 26 Hospitals, October 18, 2023-March 9, 2024
Update in
-
Effectiveness of Updated 2023-2024 (Monovalent XBB.1.5) COVID-19 Vaccination Against SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 Lineage Hospitalization and a Comparison of Clinical Severity-IVY Network, 26 Hospitals, October 18, 2023-March 9, 2024.Clin Infect Dis. 2024 Aug 6:ciae405. doi: 10.1093/cid/ciae405. Online ahead of print. Clin Infect Dis. 2024. PMID: 39107255
Abstract
Background: Assessing COVID-19 vaccine effectiveness (VE) and severity of SARS-CoV-2 variants can inform public health risk assessments and decisions about vaccine composition. BA.2.86 and its descendants, including JN.1 (referred to collectively as "JN lineages"), emerged in late 2023 and exhibited substantial genomic divergence from co-circulating XBB lineages.
Methods: We analyzed patients hospitalized with COVID-19-like illness at 26 hospitals in 20 U.S. states admitted October 18, 2023-March 9, 2024. Using a test-negative, case-control design, we estimated the effectiveness of an updated 2023-2024 (Monovalent XBB.1.5) COVID-19 vaccine dose against sequence-confirmed XBB and JN lineage hospitalization using logistic regression. Odds of severe outcomes, including intensive care unit (ICU) admission and invasive mechanical ventilation (IMV) or death, were compared for JN versus XBB lineage hospitalizations using logistic regression.
Results: 585 case-patients with XBB lineages, 397 case-patients with JN lineages, and 4,580 control-patients were included. VE in the first 7-89 days after receipt of an updated dose was 54.2% (95% CI = 36.1%-67.1%) against XBB lineage hospitalization and 32.7% (95% CI = 1.9%-53.8%) against JN lineage hospitalization. Odds of ICU admission (adjusted odds ratio [aOR] 0.80; 95% CI = 0.46-1.38) and IMV or death (aOR 0.69; 95% CI = 0.34-1.40) were not significantly different among JN compared to XBB lineage hospitalizations.
Conclusions: Updated 2023-2024 COVID-19 vaccination provided protection against both XBB and JN lineage hospitalization, but protection against the latter may be attenuated by immune escape. Clinical severity of JN lineage hospitalizations was not higher relative to XBB lineage hospitalizations.
Conflict of interest statement
Potential conflicts of interest. All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. James D. Chappell. MD, PhD reports grant support by Merck, outside the submitted work. Manjusha Gaglani, MBBS reports grants from CDC, CDC-Abt and CDC-Westat sponsored studies, serving as the Emeritus Chair of TPS IDI Committee (Co-Chair from Sept 2016 - August 2022) and ex TPS Texas RSV Taskforce Chair (May 2021- August 2022), outside the submitted work. Robert L Gottlieb, MD, PhD reports grants or contracts to his institution from AstraZeneca, Eli Lilly, Gilead, Johnson & Johnson, Pfizer, Regeneron, and Roivant Sciences (Kinevant Sciences), participation on advisory boards and/or consulting fees from AbbVie, AstraZeneca, Eli Lilly, Gilead Sciences, GSK Pharmaceuticals, and Roche, payment or honoraria for lectures/speaker from Gilead Sciences, and Pfizer (the latter unrelated to infectious diseases), travel support from Gilead Sciences, de minimis investment in AbCellera, and a gift-in-kind to his institution from Gilead Sciences to facilitate an unrelated academic-sponsored clinical trial (NCT03383419), outside the submitted work. Carlos G. Grijalva, MD MPH reports research support from CDC, NIH, AHRQ, Syneos Health and FDA, and has served in a Scientific Advisory Board for Merck, outside the submitted work. Natasha Halasa reports current funding from Merck and served on an advisory board for CSL-Seqirus, outside the submitted work. Adam S. Lauring, MD, PhD reports funding from NIAID, CDC, MDHHS, Burroughs Wellcome Fund, and consulting fees from Roche, outside the submitted work. Ithan D. Peltan reports funding from the National Institute of General Medical Studies (R35GM151147), grants from NIH and Janssen Pharmaceuticals, and funding to his institution from Regeneron and Bluejay Diagnostics, outside the submitted work. Mayur Ramesh MD reports participating as an unbranded speaker for AstraZeneca, and serving on an advisory board for Pfizer and Moderna, outside the submitted work. Ivana A. Vaughn reports grant funding from CDC via University of Michigan for US Flu VE Network, as well as eMaxHealth, outside the submitted work. Michelle Ng Gong reports funding from NIH and serving on a scientific advisory panel for Regeneron, Radiometer, Novartis, Philips Healthcare, and serving on the DSMB for clinical trials for NIH and nutrition, outside the submitted work. Catherine L. Hough, MD MSc reports funding from NIH, outside the submitted work. No other potential conflicts of interest were disclosed.
Figures
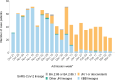


References
-
- Grant R, Sacks JA, Abraham P, Chunsuttiwat S, Cohen C, Figueroa JP, et al. When to update COVID-19 vaccine composition. Nat Med. 2023;29: 776–780. - PubMed
-
- Statement on the antigen composition of COVID-19 vaccines. [cited 2 May 2024]. Available: https://www.who.int/news/item/26-04-2024-statement-on-the-antigen-compos...
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
