Robust GRK2/3/6-dependent desensitization of oxytocin receptor in neurons
- PMID: 38883814
- PMCID: PMC11179071
- DOI: 10.1016/j.isci.2024.110047
Robust GRK2/3/6-dependent desensitization of oxytocin receptor in neurons
Abstract
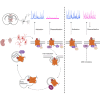
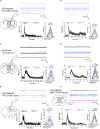
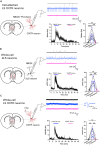
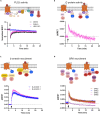
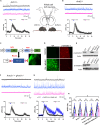
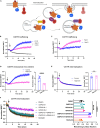
Oxytocin plays critical roles in the brain as a neuromodulator, regulating social and other affective behavior. However, the regulatory mechanisms controlling oxytocin receptor (OXTR) signaling in neurons remain unexplored. In this study, we have identified robust and rapid-onset desensitization of OXTR response in multiple regions of the mouse brain. Both cell autonomous spiking response and presynaptic activation undergo similar agonist-induced desensitization. G-protein-coupled receptor kinases (GRK) GRK2, GRK3, and GRK6 are recruited to the activated OXTR in neurons, followed by recruitment of β-arrestin-1 and -2. Neuronal OXTR desensitization was impaired by suppression of GRK2/3/6 kinase activity but remained unaltered with double knockout of β-arrestin-1 and -2. Additionally, we observed robust agonist-induced internalization of neuronal OXTR and its Rab5-dependent recruitment to early endosomes, which was impaired by GRK2/3/6 inhibition. This work defines distinctive aspects of the mechanisms governing OXTR desensitization and internalization in neurons compared to prior studies in heterologous cells.
Keywords: biological sciences; molecular neuroscience; neuroscience.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

