Topical oxygen therapy as a novel strategy to promote wound healing and control the bacteria in implantology, oral surgery and periodontology: A review
- PMID: 38883907
- PMCID: PMC11178966
- DOI: 10.1016/j.sdentj.2024.04.004
Topical oxygen therapy as a novel strategy to promote wound healing and control the bacteria in implantology, oral surgery and periodontology: A review
Abstract
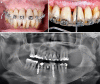
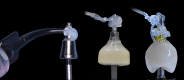
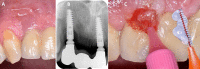
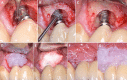
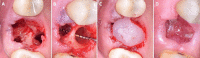
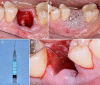
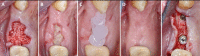
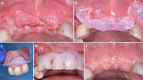
Globally, oral infections and inflammatory lesions persist as substantial public health concerns, necessitating the introduction of novel oral treatment protocols. Oral diseases are linked to various causative factors, with dental plaque/biofilm resulting from inadequate hygiene practices playing a predominant role. The strategic implementation of novel topical therapies holds promise for effectively controlling the biofilms, addressing oral infections and promoting enhanced oral wound healing. This review aims to providing a comprehensive overview of the available evidence pertaining to the potential efficacy of topical oxygen and lactoferrin-releasing biomaterials, exemplified by the blue®m formula, as novel oral care interventions within the scope of contemporary implantology, oral surgery and periodontology.
Keywords: Biomaterials; Oral biofilm; Oral care; Oxygen therapy; Peri-implantitis; Periodontitis.
© 2024 THE AUTHORS.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
Peri-implantitis in partially edentulous patients: association with inadequate plaque control.Clin Oral Implants Res. 2009 Feb;20(2):169-74. doi: 10.1111/j.1600-0501.2008.01627.x. Epub 2008 Dec 1. Clin Oral Implants Res. 2009. PMID: 19077152
-
Prevention and treatment of periodontal diseases in primary care.Evid Based Dent. 2014 Sep;15(3):68-9. doi: 10.1038/sj.ebd.6401036. Evid Based Dent. 2014. PMID: 25343386
-
Local Oxygen-Based Therapy (blue®m) for Treatment of Peri-Implant Disease: Clinical Case Presentation and Review of Literature about Conventional Local Adjunct Therapies.Medicina (Kaunas). 2024 Mar 8;60(3):447. doi: 10.3390/medicina60030447. Medicina (Kaunas). 2024. PMID: 38541173 Free PMC article. Review.
-
Principles in prevention of periodontal diseases: Consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases.J Clin Periodontol. 2015 Apr;42 Suppl 16:S5-11. doi: 10.1111/jcpe.12368. J Clin Periodontol. 2015. PMID: 25639948
-
Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis.Periodontol 2000. 2010 Jun;53:167-81. doi: 10.1111/j.1600-0757.2010.00348.x. Periodontol 2000. 2010. PMID: 20403112 Review.
Cited by
-
Management of Infected Tissues Around Dental Implants: A Short Narrative Review.Braz Dent J. 2024 Oct 25;35:e246160. doi: 10.1590/0103-6440202406160. eCollection 2024. Braz Dent J. 2024. PMID: 39476118 Free PMC article. Review.
-
Rapid Healing of Palatal Necrosis with Active Oxygen Gel: A Case Report and Management Strategy.Am J Case Rep. 2024 Oct 15;25:e945135. doi: 10.12659/AJCR.945135. Am J Case Rep. 2024. PMID: 39402814 Free PMC article.
-
Effect of active oxygen gel on the clinical parameters of MRONJ.Saudi Dent J. 2024 Nov;36(11):1456-1458. doi: 10.1016/j.sdentj.2024.09.003. Epub 2024 Sep 3. Saudi Dent J. 2024. PMID: 39619723 Free PMC article.
-
Treatment of Medication-Related Osteonecrosis of the Jaws (MRONJ) with Topical Therapy Using Active Oxygen Gel.Clin Cosmet Investig Dent. 2024 Jun 25;16:249-254. doi: 10.2147/CCIDE.S462051. eCollection 2024. Clin Cosmet Investig Dent. 2024. PMID: 38947864 Free PMC article.
-
A Novel Zinc-Containing Palatal Stent and Topical Oxygen Therapy for Wound Protection and Healing Following Mucoperiosteal Flap Surgery in the Hard Palate: A Case Report.Cureus. 2024 Jul 8;16(7):e64095. doi: 10.7759/cureus.64095. eCollection 2024 Jul. Cureus. 2024. PMID: 38979025 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

