Identification of Poly(ADP-ribose) Polymerase 9 (PARP9) as a Potent Suppressor for Mycobacterium tuberculosis Infection
- PMID: 38884060
- PMCID: PMC11169154
- DOI: 10.1007/s43657-023-00112-2
Identification of Poly(ADP-ribose) Polymerase 9 (PARP9) as a Potent Suppressor for Mycobacterium tuberculosis Infection
Abstract
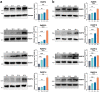
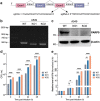
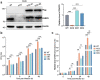
ADP-ribosylation is a reversible and dynamic post-translational modification mediated by ADP-ribosyltransferases (ARTs). Poly(ADP-ribose) polymerases (PARPs) are an important family of human ARTs. ADP-ribosylation and PARPs have crucial functions in host-pathogen interaction, especially in viral infections. However, the functions and potential molecular mechanisms of ADP-ribosylation and PARPs in Mycobacterium infection remain unknown. In this study, bioinformatics analysis revealed significantly changed expression levels of several PARPs in tuberculosis patients compared to healthy individuals. Moreover, the expression levels of these PARPs returned to normal following tuberculosis treatment. Then, the changes in the expression levels of PARPs during Mycobacterium infection were validated in Tohoku Hospital Pediatrics-1 (THP1)-induced differentiated macrophages infected with Mycobacterium model strains bacillus Calmette-Guérin (BCG) and in human lung adenocarcinoma A549 cells infected with Mycobacterium smegmatis (Ms), respectively. The mRNA levels of PARP9, PARP10, PARP12, and PARP14 were most significantly increased during infection, with corresponding increases in protein levels, indicating the possible biological functions of these PARPs during Mycobacterium infection. In addition, the biological function of host PARP9 in Mycobacterium infection was further studied. PARP9 deficiency significantly increased the infection efficiency and intracellular proliferation ability of Ms, which was reversed by the reconstruction of PARP9. Collectively, this study updates the understanding of changes in PARP expression during Mycobacterium infection and provides evidence supporting PARP9 as a potent suppressor for Mycobacterium infection.
Supplementary information: The online version contains supplementary material available at 10.1007/s43657-023-00112-2.
Keywords: Mycobacterium; Mycobacterium smegmatis; Poly(ADP-ribose) polymerase 9; Poly(ADP-ribose) polymerases; Tuberculosis.
© International Human Phenome Institutes (Shanghai) 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of InterestThe authors declare no conflict of interest.
Figures



Similar articles
-
Poly(ADP-ribose) polymerase 9 mediates early protection against Mycobacterium tuberculosis infection by regulating type I IFN production.J Clin Invest. 2023 Jun 15;133(12):e158630. doi: 10.1172/JCI158630. J Clin Invest. 2023. PMID: 37200107 Free PMC article.
-
PARPs and ADP-Ribosylation in Chronic Inflammation: A Focus on Macrophages.Pathogens. 2023 Jul 23;12(7):964. doi: 10.3390/pathogens12070964. Pathogens. 2023. PMID: 37513811 Free PMC article. Review.
-
PARP14 is regulated by the PARP9/DTX3L complex and promotes interferon γ-induced ADP-ribosylation.EMBO J. 2024 Jul;43(14):2908-2928. doi: 10.1038/s44318-024-00125-1. Epub 2024 Jun 4. EMBO J. 2024. PMID: 38834852 Free PMC article.
-
Coronavirus infection and PARP expression dysregulate the NAD metabolome: An actionable component of innate immunity.J Biol Chem. 2020 Dec 25;295(52):17986-17996. doi: 10.1074/jbc.RA120.015138. Epub 2020 Oct 13. J Biol Chem. 2020. PMID: 33051211 Free PMC article.
-
When PARPs Meet Antiviral Innate Immunity.Trends Microbiol. 2021 Sep;29(9):776-778. doi: 10.1016/j.tim.2021.01.002. Epub 2021 Jan 19. Trends Microbiol. 2021. PMID: 33483164 Review.
Cited by
-
Interactions between NAD+ metabolism and immune cell infiltration in ulcerative colitis: subtype identification and development of novel diagnostic models.Front Immunol. 2025 Feb 5;16:1479421. doi: 10.3389/fimmu.2025.1479421. eCollection 2025. Front Immunol. 2025. PMID: 39975557 Free PMC article.
-
MicrobeRX: a tool for enzymatic-reaction-based metabolite prediction in the gut microbiome.Microbiome. 2025 Mar 19;13(1):78. doi: 10.1186/s40168-025-02070-5. Microbiome. 2025. PMID: 40108657 Free PMC article.
-
Activation of NF-κB/MAPK signaling and induction of apoptosis by salicylate synthase NbtS in Nocardia farcinica promotes neuroinflammation development.mSystems. 2024 Oct 22;9(10):e0089324. doi: 10.1128/msystems.00893-24. Epub 2024 Sep 6. mSystems. 2024. PMID: 39240104 Free PMC article.
References
LinkOut - more resources
Full Text Sources
