Extracellular vesicles
- PMID: 38884207
- PMCID: PMC11304975
- DOI: 10.1093/genetics/iyae088
Extracellular vesicles
Abstract
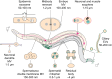
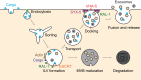
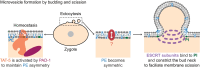
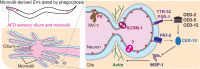
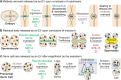
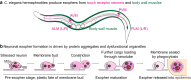
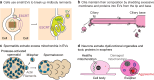
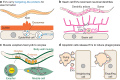
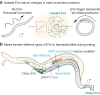
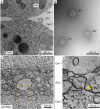
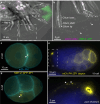
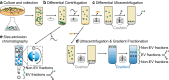
Extracellular vesicles (EVs) encompass a diverse array of membrane-bound organelles released outside cells in response to developmental and physiological cell needs. EVs play important roles in remodeling the shape and content of differentiating cells and can rescue damaged cells from toxic or dysfunctional content. EVs can send signals and transfer metabolites between tissues and organisms to regulate development, respond to stress or tissue damage, or alter mating behaviors. While many EV functions have been uncovered by characterizing ex vivo EVs isolated from body fluids and cultured cells, research using the nematode Caenorhabditis elegans has provided insights into the in vivo functions, biogenesis, and uptake pathways. The C. elegans EV field has also developed methods to analyze endogenous EVs within the organismal context of development and adult physiology in free-living, behaving animals. In this review, we summarize major themes that have emerged for C. elegans EVs and their relevance to human health and disease. We also highlight the diversity of biogenesis mechanisms, locations, and functions of worm EVs and discuss open questions and unexplored topics tenable in C. elegans, given the nematode model is ideal for light and electron microscopy, genetic screens, genome engineering, and high-throughput omics.
Keywords: Caenorhabditis elegans; cilia; exosome; extracellular vesicle; microvesicle; midbody remnant; spermatogenesis.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The author(s) declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

