Community-Based Cluster-Randomized Trial to Reduce Opioid Overdose Deaths
- PMID: 38884347
- PMCID: PMC11761538
- DOI: 10.1056/NEJMoa2401177
Community-Based Cluster-Randomized Trial to Reduce Opioid Overdose Deaths
Abstract
Background: Evidence-based practices for reducing opioid-related overdose deaths include overdose education and naloxone distribution, the use of medications for the treatment of opioid use disorder, and prescription opioid safety. Data are needed on the effectiveness of a community-engaged intervention to reduce opioid-related overdose deaths through enhanced uptake of these practices.
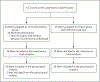
Methods: In this community-level, cluster-randomized trial, we randomly assigned 67 communities in Kentucky, Massachusetts, New York, and Ohio to receive the intervention (34 communities) or a wait-list control (33 communities), stratified according to state. The trial was conducted within the context of both the coronavirus disease 2019 (Covid-19) pandemic and a national surge in the number of fentanyl-related overdose deaths. The trial groups were balanced within states according to urban or rural classification, previous overdose rate, and community population. The primary outcome was the number of opioid-related overdose deaths among community adults.
Results: During the comparison period from July 2021 through June 2022, the population-averaged rates of opioid-related overdose deaths were similar in the intervention group and the control group (47.2 deaths per 100,000 population vs. 51.7 per 100,000 population), for an adjusted rate ratio of 0.91 (95% confidence interval, 0.76 to 1.09; P = 0.30). The effect of the intervention on the rate of opioid-related overdose deaths did not differ appreciably according to state, urban or rural category, age, sex, or race or ethnic group. Intervention communities implemented 615 evidence-based practice strategies from the 806 strategies selected by communities (254 involving overdose education and naloxone distribution, 256 involving the use of medications for opioid use disorder, and 105 involving prescription opioid safety). Of these evidence-based practice strategies, only 235 (38%) had been initiated by the start of the comparison year.
Conclusions: In this 12-month multimodal intervention trial involving community coalitions in the deployment of evidence-based practices to reduce opioid overdose deaths, death rates were similar in the intervention group and the control group in the context of the Covid-19 pandemic and the fentanyl-related overdose epidemic. (Funded by the National Institutes of Health; HCS ClinicalTrials.gov number, NCT04111939.).
Copyright © 2024 Massachusetts Medical Society.
Figures
References
-
- National Institute on Drug Abuse. Drug overdose death rates. January 20, 2022. (https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates).
-
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional drug overdose death counts. Hyattsville, MD: National Center for Health Statistics, 2024. (https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm).
-
- O’Donnell J, Tanz LJ, Miller KD, et al. Drug overdose deaths with evidence of counterfeit pill use — United States, July 2019–December 2021. MMWR Morb Mortal Wkly Rep 2023;72:949–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 DA049394/DA/NIDA NIH HHS/United States
- UM1 DA049415/DA/NIDA NIH HHS/United States
- UM1DA049415/DA/NIDA NIH HHS/United States
- UM1DA049394/DA/NIDA NIH HHS/United States
- UM1DA049412/DA/NIDA NIH HHS/United States
- UM1DA049417/DA/NIDA NIH HHS/United States
- UM1DA049406/DA/NIDA NIH HHS/United States
- UM1 DA049412/DA/NIDA NIH HHS/United States
- UM1 DA049417/DA/NIDA NIH HHS/United States
- UM1 DA049406/DA/NIDA NIH HHS/United States
- UM1DA049394/DA/NIDA NIH HHS/United States
- UM1DA049406/DA/NIDA NIH HHS/United States
- UM1DA049412/DA/NIDA NIH HHS/United States
- UM1DA049415/DA/NIDA NIH HHS/United States
- UM1DA049417/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
