Structural characterization of ligand binding and pH-specific enzymatic activity of mouse Acidic Mammalian Chitinase
- PMID: 38884443
- PMCID: PMC11182645
- DOI: 10.7554/eLife.89918
Structural characterization of ligand binding and pH-specific enzymatic activity of mouse Acidic Mammalian Chitinase
Abstract
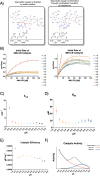
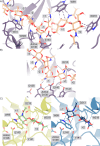
Chitin is an abundant biopolymer and pathogen-associated molecular pattern that stimulates a host innate immune response. Mammals express chitin-binding and chitin-degrading proteins to remove chitin from the body. One of these proteins, Acidic Mammalian Chitinase (AMCase), is an enzyme known for its ability to function under acidic conditions in the stomach but is also active in tissues with more neutral pHs, such as the lung. Here, we used a combination of biochemical, structural, and computational modeling approaches to examine how the mouse homolog (mAMCase) can act in both acidic and neutral environments. We measured kinetic properties of mAMCase activity across a broad pH range, quantifying its unusual dual activity optima at pH 2 and 7. We also solved high-resolution crystal structures of mAMCase in complex with oligomeric GlcNAcn, the building block of chitin, where we identified extensive conformational ligand heterogeneity. Leveraging these data, we conducted molecular dynamics simulations that suggest how a key catalytic residue could be protonated via distinct mechanisms in each of the two environmental pH ranges. These results integrate structural, biochemical, and computational approaches to deliver a more complete understanding of the catalytic mechanism governing mAMCase activity at different pH. Engineering proteins with tunable pH optima may provide new opportunities to develop improved enzyme variants, including AMCase, for therapeutic purposes in chitin degradation.
Keywords: E. coli; biochemistry; chemical biology; chitin; enzyme; human; lung; molecular biophysics; mouse; structural biology.
© 2023, Díaz et al.
Conflict of interest statement
RD, AE, GC, PA, IY, BF, MT, IS No competing interests declared, SV, RL S.J.V.D. and R.M.L. are listed as inventors on a patent United States application: 17/505,561 for the use of chitinases to treat fibrotic lung disease. S.J.V.D., R.M.L., and J.S.F. are listed as inventors on a patent for mutant chitinases with enhanced expression and activity, JF S.J.V.D. and R.M.L. are listed as inventors on a patent for the use of chitinases to treat fibrotic lung disease. United States application: 17/505,561 S.J.V.D., R.M.L., and J.S.F. are listed as inventors on a patent for mutant chitinases with enhanced expression and activity
Figures











Update of
-
Structural characterization of ligand binding and pH-specific enzymatic activity of mouse Acidic Mammalian Chitinase.bioRxiv [Preprint]. 2024 Mar 25:2023.06.03.542675. doi: 10.1101/2023.06.03.542675. bioRxiv. 2024. Update in: Elife. 2024 Jun 17;12:RP89918. doi: 10.7554/eLife.89918. PMID: 37398339 Free PMC article. Updated. Preprint.
References
-
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica. Section D, Biological Crystallography. 2012;68:352–367. doi: 10.1107/S0907444912001308. - DOI - PMC - PubMed
-
- Aguilera B, Ghauharali-van der Vlugt K, Helmond MTJ, Out JMM, Donker-Koopman WE, Groener JEM, Boot RG, Renkema GH, van der Marel GA, van Boom JH, Overkleeft HS, Aerts J. Transglycosidase activity of chitotriosidase: improved enzymatic assay for the human macrophage chitinase. The Journal of Biological Chemistry. 2003;278:40911–40916. doi: 10.1074/jbc.M301804200. - DOI - PubMed
-
- Bhunia B, Dutta D, Chaudhuri S. Extracellular alkaline protease from Bacillus licheniformis NCIM‐2042: Improving enzyme activity assay and characterization. Engineering in Life Sciences. 2011;11:207–215. doi: 10.1002/elsc.201000020. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

