Clinical Presentation of Early Syphilis and Genomic Sequences of Treponema pallidum Strains in Patient Specimens and Isolates Obtained by Rabbit Inoculation
- PMID: 38884588
- PMCID: PMC11646597
- DOI: 10.1093/infdis/jiae322
Clinical Presentation of Early Syphilis and Genomic Sequences of Treponema pallidum Strains in Patient Specimens and Isolates Obtained by Rabbit Inoculation
Abstract
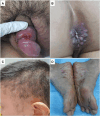
Background: The global resurgence of syphilis necessitates vaccine development.
Methods: We collected ulcer exudates and blood from 17 participants with primary syphilis (PS) and skin biopsies and blood from 51 patients with secondary syphilis (SS) in Guangzhou, China, for Treponema pallidum subsp pallidum (TPA) quantitative polymerase chain reaction, whole genome sequencing (WGS), and isolation of TPA in rabbits.
Results: TPA DNA was detected in 15 of 17 ulcer exudates and 3 of 17 blood PS specimens. TPA DNA was detected in 50 of 51 SS skin biopsies and 27 of 51 blood specimens. TPA was isolated from 47 rabbits with success rates of 71% (12/17) and 69% (35/51), respectively, from ulcer exudates and SS bloods. We obtained paired genomic sequences from 24 clinical samples and corresponding rabbit isolates. Six SS14- and 2 Nichols-clade genome pairs contained rare discordances. Forty-one of the 51 unique TPA genomes clustered within SS14 subgroups largely from East Asia, while 10 fell into Nichols C and E subgroups.
Conclusions: Our TPA detection rate was high from PS ulcer exudates and SS skin biopsies and over 50% from SS blood, with TPA isolation in more than two-thirds of samples. Our results support the use of WGS from rabbit isolates to inform vaccine development.
Keywords: Treponema pallidum; polA qPCR; chancre; early syphilis; genomic sequences; primary syphilis; rabbit inoculation; secondary syphilis.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. B. P. reports research support from Gilead Sciences, nonfinancial support from Abbott Laboratories, and consulting for Zymeron Corporation outside the scope of the current manuscript. J. D. R. has licensing agreements for recombinant TPA proteins as syphilis serodiagnostic reagents with Biokit SA, Chembio, and Span Diagnostics. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



Comment in
-
Syphilis on the rise - a need for alternative therapies and vaccines.Nat Rev Urol. 2024 Aug;21(8):457. doi: 10.1038/s41585-024-00916-5. Nat Rev Urol. 2024. PMID: 39014023 No abstract available.
References
-
- Zhang L. The Health Commission of Guangdong Province announced the incidence of notifiable infectious diseases in the province for 2021 and January 2022. Guangdong Province, China, 2022.
-
- Taouk ML, Taiaroa G, Pasricha S, et al. Characterisation of Treponema pallidum lineages within the contemporary syphilis outbreak in Australia: a genomic epidemiological analysis. Lancet Microbe 2022; 3:e417–26. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

