Proofreading mechanisms of the innate immune receptor RIG-I: distinguishing self and viral RNA
- PMID: 38884803
- PMCID: PMC11346460
- DOI: 10.1042/BST20230724
Proofreading mechanisms of the innate immune receptor RIG-I: distinguishing self and viral RNA
Abstract
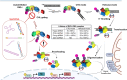
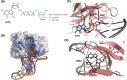
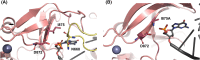
The RIG-I-like receptors (RLRs), comprising retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated gene 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2), are pattern recognition receptors belonging to the DExD/H-box RNA helicase family of proteins. RLRs detect viral RNAs in the cytoplasm and respond by initiating a robust antiviral response that up-regulates interferon and cytokine production. RIG-I and MDA5 complement each other by recognizing different RNA features, and LGP2 regulates their activation. RIG-I's multilayered RNA recognition and proofreading mechanisms ensure accurate viral RNA detection while averting harmful responses to host RNAs. RIG-I's C-terminal domain targets 5'-triphosphate double-stranded RNA (dsRNA) blunt ends, while an intrinsic gating mechanism prevents the helicase domains from non-specifically engaging with host RNAs. The ATPase and RNA translocation activity of RIG-I adds another layer of selectivity by minimizing the lifetime of RIG-I on non-specific RNAs, preventing off-target activation. The versatility of RIG-I's ATPase function also amplifies downstream signaling by enhancing the signaling domain (CARDs) exposure on 5'-triphosphate dsRNA and promoting oligomerization. In this review, we offer an in-depth understanding of the mechanisms RIG-I uses to facilitate viral RNA sensing and regulate downstream activation of the immune system.
Keywords: RIG-I like receptors; helicase; nucleic acid receptors.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
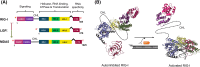




Similar articles
-
Unraveling blunt-end RNA binding and ATPase-driven translocation activities of the RIG-I family helicase LGP2.Nucleic Acids Res. 2024 Jan 11;52(1):355-369. doi: 10.1093/nar/gkad1106. Nucleic Acids Res. 2024. PMID: 38015453 Free PMC article.
-
Solution structures of cytosolic RNA sensor MDA5 and LGP2 C-terminal domains: identification of the RNA recognition loop in RIG-I-like receptors.J Biol Chem. 2009 Jun 26;284(26):17465-74. doi: 10.1074/jbc.M109.007179. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380577 Free PMC article.
-
Structures of RIG-I-Like Receptors and Insights into Viral RNA Sensing.Adv Exp Med Biol. 2019;1172:157-188. doi: 10.1007/978-981-13-9367-9_8. Adv Exp Med Biol. 2019. PMID: 31628656 Review.
-
LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses.Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1512-7. doi: 10.1073/pnas.0912986107. Epub 2010 Jan 8. Proc Natl Acad Sci U S A. 2010. PMID: 20080593 Free PMC article.
-
Sensing of viral nucleic acids by RIG-I: from translocation to translation.Eur J Cell Biol. 2012 Jan;91(1):78-85. doi: 10.1016/j.ejcb.2011.01.015. Epub 2011 Apr 14. Eur J Cell Biol. 2012. PMID: 21496944 Free PMC article. Review.
Cited by
-
Rational Design and Immunological Mechanisms of Circular RNA-Based Vaccines: Emerging Frontiers in Combating Pathogen Infection.Vaccines (Basel). 2025 May 26;13(6):563. doi: 10.3390/vaccines13060563. Vaccines (Basel). 2025. PMID: 40573894 Free PMC article. Review.
-
The Role of Pattern Recognition Receptors in Epigenetic and Metabolic Reprogramming: Insights into Trained Immunity.J Inflamm Res. 2025 Jun 13;18:7795-7811. doi: 10.2147/JIR.S513325. eCollection 2025. J Inflamm Res. 2025. PMID: 40535352 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

