Exploring laser-induced acute and chronic retinal vein occlusion mouse models: Development, temporal in vivo imaging, and application perspectives
- PMID: 38885229
- PMCID: PMC11182531
- DOI: 10.1371/journal.pone.0305741
Exploring laser-induced acute and chronic retinal vein occlusion mouse models: Development, temporal in vivo imaging, and application perspectives
Abstract
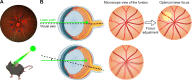
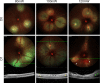
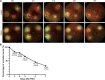
Photodynamic venous occlusion is a commonly accepted method for establishing mouse models of retinal vein occlusion (RVO). However, existing model parameters do not distinguish between acute and chronic RVO subtypes. Large variations in laser energy seem to correlate with fluctuating retinopathy severity and high rates of venous recanalization during the acute phase, along with the variable levels of retinal perfusion during the chronic phase. After optimizing the modeling procedure and defining success and exclusion criteria, laser energy groups of 80mW, 100mW, and 120mW were established. Multimodal imaging confirmed that higher energy levels increased the incidence of retinal cystoid edema and intraretinal hemorrhage, exacerbated the severity of exudative retinal detachment, and reduced the venous recanalization rate. For the acute model, 100mW was considered an appropriate parameter for balancing moderate retinopathy and venous recanalization. Continuous imaging follow-up revealed that day 1 after RVO was the optimal observation point for peaking of retinal thickness and intensive occurrence of retinal cystic edema and intraretinal hemorrhage. After excluding the influence of venous recanalization on retinal thickness, acute retinal edema demonstrated a positive response to standard anti-vascular endothelial growth factor therapy, validating the clinical relevance of the acute RVO model for further study in pathogenic mechanisms and therapeutic efficacy. For the chronic model, the 120mW parameter with the lowest venous recanalization rate was applied, accompanied by an increase in both photocoagulation shots and range to ensure sustained vein occlusion. Imaging follow-up clarified non-ischemic retinopathy characterized by tortuosity and dilation of the distal end, branches, and adjacent veins of the occluded vein. These morphological changes are quantifiable and could be combined with electrophysiological functional assessment for treatment effectiveness evaluation. Moreover, the stable state of venous occlusion may facilitate investigations into response and compensation mechanisms under conditions of chronic retinal hypoperfusion.
Copyright: © 2024 Xu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Aldosterone Exposure Causes Increased Retinal Edema and Severe Retinopathy Following Laser-Induced Retinal Vein Occlusion in Mice.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3355-3365. doi: 10.1167/iovs.17-23073. Invest Ophthalmol Vis Sci. 2018. PMID: 30025072
-
Creation of Retinal Vein Occlusion Model in Cynomolgus Monkeys and Determination of its Pathological Features.Curr Neurovasc Res. 2021;18(1):123-133. doi: 10.2174/1567202617999200831151118. Curr Neurovasc Res. 2021. PMID: 32867658
-
Investigation of retinal morphology alterations using spectral domain optical coherence tomography in a mouse model of retinal branch and central retinal vein occlusion.PLoS One. 2015 Mar 16;10(3):e0119046. doi: 10.1371/journal.pone.0119046. eCollection 2015. PLoS One. 2015. PMID: 25775456 Free PMC article.
-
[Ischemia and laser photocoagulation in retinal vein occlusion].Ophthalmologie. 2022 Nov;119(11):1121-1128. doi: 10.1007/s00347-022-01750-z. Epub 2022 Nov 10. Ophthalmologie. 2022. PMID: 36357589 Review. German.
-
Ischemic retinal vein occlusion: characterizing the more severe spectrum of retinal vein occlusion.Surv Ophthalmol. 2018 Nov-Dec;63(6):816-850. doi: 10.1016/j.survophthal.2018.04.005. Epub 2018 Apr 27. Surv Ophthalmol. 2018. PMID: 29705175 Review.
Cited by
-
OculusNet: Detection of retinal diseases using a tailored web-deployed neural network and saliency maps for explainable AI.Front Med (Lausanne). 2025 Jul 2;12:1596726. doi: 10.3389/fmed.2025.1596726. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40672824 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources

