Comparative proteomics reveals that fatty acid metabolism is involved in myocardial adaptation to chronic hypoxic injury
- PMID: 38885281
- PMCID: PMC11182518
- DOI: 10.1371/journal.pone.0305571
Comparative proteomics reveals that fatty acid metabolism is involved in myocardial adaptation to chronic hypoxic injury
Abstract
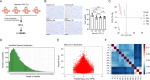
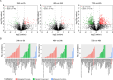
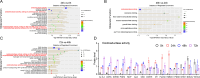
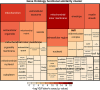
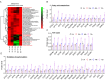
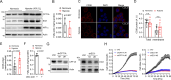
Congenital heart disease (CHD) is the most serious form of heart disease, and chronic hypoxia is the basic physiological process underlying CHD. Some patients with CHD do not undergo surgery, and thus, they remain susceptible to chronic hypoxia, suggesting that some protective mechanism might exist in CHD patients. However, the mechanism underlying myocardial adaptation to chronic hypoxia remains unclear. Proteomics was used to identify the differentially expressed proteins in cardiomyocytes cultured under hypoxia for different durations. Western blotting assays were used to verify protein expression. A Real-Time Cell Analyzer (RTCA) was used to analyze cell growth. In this study, 3881 proteins were identified by proteomics. Subsequent bioinformatics analysis revealed that proteins were enriched in regulating oxidoreductase activity. Functional similarity cluster analyses showed that chronic hypoxia resulted in proteins enrichment in the mitochondrial metabolic pathway. Further KEGG analyses found that the proteins involved in fatty acid metabolism, the TCA cycle and oxidative phosphorylation were markedly upregulated. Moreover, knockdown of CPT1A or ECI1, which is critical for fatty acid degradation, suppressed the growth of cardiomyocytes under chronic hypoxia. The results of our study revealed that chronic hypoxia activates fatty acid metabolism to maintain the growth of cardiomyocytes.
Copyright: © 2024 Chen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
PGC-1α Participates in the Protective Effect of Chronic Intermittent Hypobaric Hypoxia on Cardiomyocytes.Cell Physiol Biochem. 2018;50(5):1891-1902. doi: 10.1159/000494869. Epub 2018 Nov 5. Cell Physiol Biochem. 2018. PMID: 30396162
-
Carnitine palmitoyltransferase 1A functions to repress FoxO transcription factors to allow cell cycle progression in ovarian cancer.Oncotarget. 2016 Jan 26;7(4):3832-46. doi: 10.18632/oncotarget.6757. Oncotarget. 2016. PMID: 26716645 Free PMC article.
-
iTRAQ-Based Proteomic Analysis Reveals Recovery of Impaired Mitochondrial Function in Ischemic Myocardium by Shenmai Formula.J Proteome Res. 2018 Feb 2;17(2):794-803. doi: 10.1021/acs.jproteome.7b00450. Epub 2018 Jan 23. J Proteome Res. 2018. PMID: 29300489
-
PHDs/CPT1B/VDAC1 axis regulates long-chain fatty acid oxidation in cardiomyocytes.Cell Rep. 2021 Oct 5;37(1):109767. doi: 10.1016/j.celrep.2021.109767. Cell Rep. 2021. PMID: 34610308 Free PMC article.
-
Suppression of Myocardial Hypoxia-Inducible Factor-1α Compromises Metabolic Adaptation and Impairs Cardiac Function in Patients With Cyanotic Congenital Heart Disease During Puberty.Circulation. 2021 Jun 8;143(23):2254-2272. doi: 10.1161/CIRCULATIONAHA.120.051937. Epub 2021 Mar 5. Circulation. 2021. PMID: 33663226
Cited by
-
Unraveling the Translational Relevance of β-Hydroxybutyrate as an Intermediate Metabolite and Signaling Molecule.Int J Mol Sci. 2025 Jul 30;26(15):7362. doi: 10.3390/ijms26157362. Int J Mol Sci. 2025. PMID: 40806491 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

