Occurrence of premature battery depletion in a large multicentre registry of subcutaneous cardioverter-defibrillator patients
- PMID: 38885309
- PMCID: PMC11218560
- DOI: 10.1093/europace/euae170
Occurrence of premature battery depletion in a large multicentre registry of subcutaneous cardioverter-defibrillator patients
Abstract
Aims: Subcutaneous implantable cardioverter-defibrillators (S-ICDs) have become established in preventing sudden cardiac death, with some advantages over transvenous defibrillator systems, including a lower incidence of lead failures. Despite technological advancements, S-ICD carriers may suffer from significant complications, such as premature battery depletion (PBD), that led to an advisory for nearly 40 000 patients. This multicentre study evaluated the incidence of PBD in a large set of S-ICD patients.
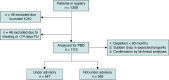
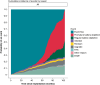
Methods and results: Data from patients implanted with S-ICD models A209 and A219 between October 2012 and July 2023 across nine centres in Europe and the USA were reviewed. Incidence and implications of PBD, defined as clinically observed sudden drop in battery longevity, were analysed and compared to PBD with the definition of battery depletion within 60 months. Prospectively collected clinical data were obtained retrospectively from medical records, device telemetry, and manufacturer reports. This registry is listed on ClinicalTrials.gov (NCT05713708). Of the 1112 S-ICD devices analysed, 547 (49.2%) were equipped with a potentially affected capacitor linked to PBD occurrence, currently under Food and Drug Administration advisory. The median follow-up time for all patients was 46 [inter-quartile range (IQR) 24-63] months. Clinically suspected PBD was observed in 159 (29.1%) of cases, with a median time to generator removal or replacement of 65 (IQR 55-72) months, indicative of significant deviations from expected battery lifespan. Manufacturer confirmation of PBD was made in 91.7% of devices returned for analysis. No cases of PBD were observed in devices that were not under advisory.
Conclusion: This manufacturer-independent analysis highlights a notable incidence of PBD in patients equipped with S-ICD models under advisory, and the rate of PBD in this study corresponds to the rate currently estimated by the manufacturer. To the best of our knowledge, this provides the largest contemporary peer-reviewed study cohort investigating the actual incidence of PBD in S-ICD patients. These findings emphasize the importance of post-market registries in collaboration between clinicians and the manufacturer to optimize safety and efficacy in S-ICD treatment.
Keywords: Battery depletion; Device malfunction; Multicentre registry; Premature battery depletion; S-ICD; Subcutaneous implantable cardioverter-defibrillator.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: J.W. reports having received lecture fees from Abbott and Boston Scientific and educational fees from Boston Scientific and Johnson & Johnson. J.L. and D.S. report having received lecture fees from Johnson & Johnson, Abbott, and Boston Scientific. A.S. reports having received lecture fees from Medtronic, Boston Scientific, Abbott, and Johnson & Johnson. J.H.-L. reports having received educational fees from Boston Scientific, Medtronic, Abbott, and Biotronik and speaker fees from Abbott. N.T.S. reports having received research funding from Abbott. N.B. reports having received an educational grant from Biotronik and speaker fees from Medtronic and Abbott, all outside this submitted work. A.B. reports having received consultant and/or speaker fees from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cook Medical, Daiichi Sankyo, Medtronic, Pfizer, and Spectranetics/Philips. J.S. reports having received educational fees from Boston Scientific and Johnson & Johnson and lecture fees from Abbott. All other authors report nothing to declare.
Figures

References
-
- Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA et al. ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Hear J 2022;43:3997–4126. - PubMed
-
- Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, Jordaens L et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med 2010;363:36–44. - PubMed
-
- Boveda S, Lenarczyk R, Haugaa K, Fumagalli S, Madrid AH, Defaye P et al. Implantation of subcutaneous implantable cardioverter defibrillators in Europe: results of the European Heart Rhythm Association survey. Europace 2016;18:1434–9. - PubMed
-
- De Veld JA, Delnoy PPHM, Olde Nordkamp LRA, Kuschyk J, Bonnemeier H, Bijsterveld NR et al. Subcutaneous ICD therapy results in a similar quality of life compared with transvenous ICD therapy during both short- and long-term follow-up. Europace 2023;25:euad122.427.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

