Relationships between PET and blood plasma biomarkers in corticobasal syndrome
- PMID: 38885334
- PMCID: PMC11247700
- DOI: 10.1002/alz.13914
Relationships between PET and blood plasma biomarkers in corticobasal syndrome
Abstract
Introduction: Corticobasal syndrome (CBS) can result from underlying Alzheimer's disease (AD) pathologies. Little is known about the utility of blood plasma metrics to predict positron emission tomography (PET) biomarker-confirmed AD in CBS.
Methods: A cohort of eighteen CBS patients (8 amyloid beta [Aβ]+; 10 Aβ-) and 8 cognitively unimpaired (CU) individuals underwent PET imaging and plasma analysis. Plasma concentrations were compared using a Kruskal-Wallis test. Spearman correlations assessed relationships between plasma concentrations and PET uptake.
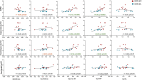
Results: CBS Aβ+ group showed a reduced Aβ42/40 ratio, with elevated phosphorylated tau (p-tau)181, glial fibrillary acidic protein (GFAP), and neurofilament light (NfL) concentrations, while CBS Aβ- group only showed elevated NfL concentration compared to CU. Both p-tau181 and GFAP were able to differentiate CBS Aβ- from CBS Aβ+ and showed positive associations with Aβ and tau PET uptake.
Discussion: This study supports use of plasma p-tau181 and GFAP to detect AD in CBS. NfL shows potential as a non-specific disease biomarker of CBS regardless of underlying pathology.
Highlights: Plasma phosphorylated tau (p-tau)181 and glial fibrillary acidic protein (GFAP) concentrations differentiate corticobasal syndrome (CBS) amyloid beta (Aβ)- from CBS Aβ+. Plasma neurofilament light concentrations are elevated in CBS Aβ- and Aβ+ compared to controls. Plasma p-tau181 and GFAP concentrations were associated with Aβ and tau positron emission tomography (PET) uptake. Aβ42/40 ratio showed a negative correlation with Aβ PET uptake.
Keywords: blood plasma biomarkers; corticobasal syndrome; positron emission tomography uptake.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Singh and Alla Alnobani have no disclosures to report. Dr. Whitwell, Dr. Machulda, Dr. Schwarz, and Dr. Josephs reports receiving research funding from the NIH. Dr. Graff‐Radford reports receiving research support from the NIH and DSMB for StrokeNET. He is an investigator in a trial sponsored by USC and EISAI. Dr. Kanekiyo reports receiving research funding from the NIH and Cure Alzheimer's Fund. Matthew Senjem reports holding stock in Gilead Sciences, Inc., Inovio Pharmaceuticals, Medtronic, Oncothyreon, Inc., and PAREXEL International. Dr. Jack reports serving on an independent data monitoring board for Roche, has consulted for and served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from NIH, the GHR foundation, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic. Dr. Lowe reports consulting for Bayer Schering Pharma, Piramal Life Sciences, Life Molecular Imaging, Eisai Inc., AVID Radiopharmaceuticals, and Merck Research and receiving research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH (NIA, NCI). Dr. Mielke reports consulting for Biogen, Eisai, Lilly, Merck, Roche, and Siemens Healthineers and receives research support from the NIH, Department of Defense, and Alzheimer's Association. Author disclosures are available in the supporting information.
Figures
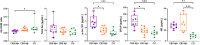
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

