The IFITM5 mutation in osteogenesis imperfecta type V is associated with an ERK/SOX9-dependent osteoprogenitor differentiation defect
- PMID: 38885336
- PMCID: PMC11290974
- DOI: 10.1172/JCI170369
The IFITM5 mutation in osteogenesis imperfecta type V is associated with an ERK/SOX9-dependent osteoprogenitor differentiation defect
Abstract
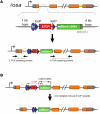
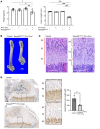
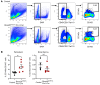
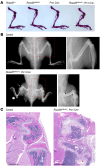
Osteogenesis imperfecta (OI) type V is the second most common form of OI, distinguished by hyperplastic callus formation and calcification of the interosseous membranes, in addition to the bone fragility. It is caused by a recurrent, dominant pathogenic variant (c.-14C>T) in interferon-induced transmembrane protein 5 (IFITM5). Here, we generated a conditional Rosa26-knockin mouse model to study the mechanistic consequences of the recurrent mutation. Expression of the mutant Ifitm5 in osteo-chondroprogenitor or chondrogenic cells resulted in low bone mass and growth retardation. Mutant limbs showed impaired endochondral ossification, cartilage overgrowth, and abnormal growth plate architecture. The cartilage phenotype correlates with the pathology reported in patients with OI type V. Surprisingly, expression of mutant Ifitm5 in mature osteoblasts caused no obvious skeletal abnormalities. In contrast, earlier expression in osteo-chondroprogenitors was associated with an increase in the skeletal progenitor cell population within the periosteum. Lineage tracing showed that chondrogenic cells expressing the mutant Ifitm5 had decreased differentiation into osteoblastic cells in diaphyseal bone. Moreover, mutant IFITM5 disrupted early skeletal homeostasis in part by activating ERK signaling and downstream SOX9 protein, and inhibition of these pathways partially rescued the phenotype in mutant animals. These data identify the contribution of a signaling defect altering osteo-chondroprogenitor differentiation as a driver in the pathogenesis of OI type V.
Keywords: Bone biology; Bone development; Bone disease; Cartilage; Genetics.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- P30 CA093373/CA/NCI NIH HHS/United States
- T32 GM007526/GM/NIGMS NIH HHS/United States
- T32 HL092332/HL/NHLBI NIH HHS/United States
- R01 AR072018/AR/NIAMS NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- R01 DE031162/DE/NIDCR NIH HHS/United States
- S10 RR024574/RR/NCRR NIH HHS/United States
- S10 OD028648/OD/NIH HHS/United States
- T32 DK007664/DK/NIDDK NIH HHS/United States
- P50 HD103555/HD/NICHD NIH HHS/United States
- R01 DE031288/DE/NIDCR NIH HHS/United States
- S10 OD016167/OD/NIH HHS/United States
- UL1 TR002369/TR/NCATS NIH HHS/United States
- U54 AR068069/AR/NIAMS NIH HHS/United States
- U54 HD083092/HD/NICHD NIH HHS/United States
- U54 HD061221/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

