Weight-Loss Endoscopy Trial: A Multicenter, Randomized, Controlled Trial Comparing Weight Loss in Endoscopically Implanted Duodenal-Jejunal Bypass Liners versus Intragastric Balloons versus a Sham Procedure
- PMID: 38885635
- PMCID: PMC11633907
- DOI: 10.1159/000539816
Weight-Loss Endoscopy Trial: A Multicenter, Randomized, Controlled Trial Comparing Weight Loss in Endoscopically Implanted Duodenal-Jejunal Bypass Liners versus Intragastric Balloons versus a Sham Procedure
Abstract
Introduction: Obesity is associated with reduced life expectancy and various comorbidities. Surgical interventions are effective but accompanied by the risk of serious complications. Less invasive endoscopic procedures mainly comprise the intragastric balloon (IB) and the duodenal-jejunal bypass liner (DJBL). A randomized, sham-controlled study comparing both procedures has not been undertaken so far.
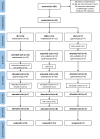
Methods: We performed a randomized, patient- and assessor-blinded, controlled trial comparing weight loss in IB versus DJBL versus a sham procedure (2:2:1 ratio). Patients with a BMI >35 kg/m2 or >30 with obesity-related comorbidities were included. The IB was removed after 6 months and the DJBL after 12 months. The main objective was successful weight loss (>10% from baseline) 12 months after explantation of the devices. Secondary outcomes were changes in comorbidities, quality of life, and complications.
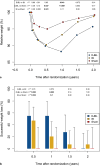
Results: Thirty-three patients were randomized. Recruitment has to be stopped suddenly in after the DJBL device lost its CE mark in Europe. In all, 11 patients received DJBL, 15 IB, and 7 were allocated to the sham group. Blinding was feasible in all patients. Weight decreased from baseline until explantation (DJBL: 129.4 ± 28.3 kg to 107.4 ± 16.7 kg; IB: 118.3 ± 22.8 kg to 107.4 ± 25.7 kg; sham: 134.6 ± 18.0 kg to 131.2 ± 14.3 kg), but patients regained weight almost to the baseline level 12 months after explantation. Only 1 patient in IB group reached the primary endpoint. Severe device-related complications were very rare.
Conclusion: Endoscopic bariatric procedures failed to achieve effective weight loss 12 months after explantation of the devices. The results of this trial need to be interpreted with caution due to its early termination.
Keywords: Bypass liner; Diabetes; Endoscopy; Intragastric balloon; Obesity; Weight loss.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare no conflicts of interest related to this study.
Figures
References
-
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32(9):1431–7. - PubMed
-
- Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–209. - PubMed
-
- Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. . Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–50. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

