Adaptive gene expression of alternative splicing variants of PGC-1α regulates whole-body energy metabolism
- PMID: 38885788
- PMCID: PMC11254180
- DOI: 10.1016/j.molmet.2024.101968
Adaptive gene expression of alternative splicing variants of PGC-1α regulates whole-body energy metabolism
Abstract
The transcriptional coactivator PGC-1α has been implicated in the regulation of multiple metabolic processes. However, the previously reported metabolic phenotypes of mice deficient in PGC-1α have been inconsistent. PGC-1α exists as multiple isoforms, including variants transcribed from an alternative first exon. We show here that alternative PGC-1α variants are the main entity that increases PGC-1α during exercise. These variants, unlike the canonical isoform of PGC-1α, are robustly upregulated in human skeletal muscle after exercise. Furthermore, the extent of this upregulation correlates with oxygen consumption. Mice lacking these variants manifest impaired energy expenditure during exercise, leading to the development of obesity and hyperinsulinemia. The alternative variants are also upregulated in brown adipose tissue in response to cold exposure, and mice lacking these variants are intolerant of a cold environment. Our findings thus indicate that an increase in PGC-1α expression, attributable mostly to upregulation of alternative variants, is pivotal for adaptive enhancement of energy expenditure and heat production and thereby essential for the regulation of whole-body energy metabolism.
Keywords: Diabetes; Energy expenditure; Exercise; Obesity; PGC-1α; Skeletal muscle.
Copyright © 2024 The Authors. Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

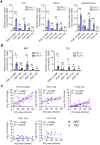
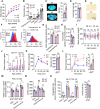
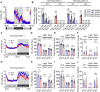

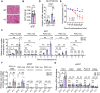
References
-
- Patti M.E., Butte A.J., Crunkhorn S., Cusi K., Berria R., Kashyap S., et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. - DOI - PMC - PubMed
-
- Kristensen J.M., Skov V., Petersson S.J., Ørtenblad N., Wojtaszewski Jørgen.F.P., Beck-Nielsen H., et al. A PGC-1α- and muscle fibre type-related decrease in markers of mitochondrial oxidative metabolism in skeletal muscle of humans with inherited insulin resistance. Diabetologia. 2014;57(5):1006–1015. doi: 10.1007/s00125-014-3187-y. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

