Synthesis of guidance available for assessing methodological quality and grading of evidence from qualitative research to inform clinical recommendations: a systematic literature review
- PMID: 38886002
- PMCID: PMC11184179
- DOI: 10.1136/rmdopen-2023-004032
Synthesis of guidance available for assessing methodological quality and grading of evidence from qualitative research to inform clinical recommendations: a systematic literature review
Abstract
Objective: To understand (1) what guidance exists to assess the methodological quality of qualitative research; (2) what methods exist to grade levels of evidence from qualitative research to inform recommendations within European Alliance of Associations for Rheumatology (EULAR).
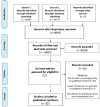
Methods: A systematic literature review was performed in multiple databases including PubMed/Medline, EMBASE, Web of Science, COCHRANE and PsycINFO, from inception to 23 October 2020. Eligible studies included primary articles and guideline documents available in English, describing the: (1) development; (2) application of validated tools (eg, checklists); (3) guidance on assessing methodological quality of qualitative research and (4) guidance on grading levels of qualitative evidence. A narrative synthesis was conducted to identify key similarities between included studies.
Results: Of 9073 records retrieved, 51 went through to full-manuscript review, with 15 selected for inclusion. Six articles described methodological tools to assess the quality of qualitative research. The tools evaluated research design, recruitment, ethical rigour, data collection and analysis. Seven articles described one approach, focusing on four key components to determine how much confidence to place in findings from systematic reviews of qualitative research. Two articles focused on grading levels of clinical recommendations based on qualitative evidence; one described a qualitative evidence hierarchy, and another a research pyramid.
Conclusion: There is a lack of consensus on the use of tools, checklists and approaches suitable for appraising the methodological quality of qualitative research and the grading of qualitative evidence to inform clinical practice. This work is expected to facilitate the inclusion of qualitative evidence in the process of developing recommendations at EULAR level.
Keywords: Health services research; Patient Reported Outcome Measures; Qualitative research; Quality Indicators, Health Care.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: EN has received speaker honoraria/participated in advisory boards for Celltrion, Pfizer, Sanofi, Gilead, Galapagos, AbbVie, Fresenius-Kabi, Lilly and holds research grants from Pfizer and Lilly.
Figures
Similar articles
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Applying GRADE-CERQual to qualitative evidence synthesis findings-paper 3: how to assess methodological limitations.Implement Sci. 2018 Jan 25;13(Suppl 1):9. doi: 10.1186/s13012-017-0690-9. Implement Sci. 2018. PMID: 29384078 Free PMC article.
-
Access to and sustainability of abortion services: a systematic review and meta-analysis for the National Institute of Health and Care Excellence-new clinical guidelines for England.Hum Reprod Update. 2020 Nov 1;26(6):886-903. doi: 10.1093/humupd/dmaa026. Hum Reprod Update. 2020. PMID: 32712660
-
Tools used to assess the quality of peer review reports: a methodological systematic review.BMC Med Res Methodol. 2019 Mar 6;19(1):48. doi: 10.1186/s12874-019-0688-x. BMC Med Res Methodol. 2019. PMID: 30841850 Free PMC article.
Cited by
-
Exploring patient activation and self-management experiences in adults with fibromyalgia: a qualitative evidence synthesis.Rheumatol Adv Pract. 2025 Mar 10;9(2):rkaf025. doi: 10.1093/rap/rkaf025. eCollection 2025. Rheumatol Adv Pract. 2025. PMID: 40093347 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources