Exploring different objectives in non-inferiority trials
- PMID: 38886014
- PMCID: PMC11181107
- DOI: 10.1136/bmj-2023-078000
Exploring different objectives in non-inferiority trials
Abstract
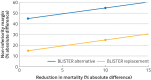
Non-inferiority trials compare the efficacy of a new treatment with an existing one where the new treatment is expected to have broadly similar efficacy to the existing treatment, but where other benefits might make the new treatment desirable. These trials might aim to demonstrate that a new treatment is either an alternative to, or a replacement for, the current treatment. In this article, how treatment comparisons can be based only on efficacy, or on both efficacy and other benefits, is explained, and guidance on how to choose the correct objective for a trial is given. This choice should influence the design of the trial (eg, choosing the non-inferiority margin and secondary outcomes), analysis, and reporting of the trial. Most non-inferiority trials aim to show only that a new treatment is an alternative to the standard of care. Being more transparent about the trial objective, however, could mean that more trials are conducted with an emphasis on the risk-benefit trade-off for a new treatment and generate more clinically meaningful trial results with a greater effect on practice.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: core support from the Medical Research Council (MRC) UK to the MRC Clinical Trials Unit for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
