EBV promotes TCR-T-cell therapy resistance by inducing CD163+M2 macrophage polarization and MMP9 secretion
- PMID: 38886114
- PMCID: PMC11184188
- DOI: 10.1136/jitc-2023-008375
EBV promotes TCR-T-cell therapy resistance by inducing CD163+M2 macrophage polarization and MMP9 secretion
Abstract
Background: Epstein-Barr virus (EBV) is a double-stranded DNA oncogenic virus. Several types of solid tumors, such as nasopharyngeal carcinoma, EBV-associated gastric carcinoma, and lymphoepithelioma-like carcinoma of the lung, have been linked to EBV infection. Currently, several TCR-T-cell therapies for EBV-associated tumors are in clinical trials, but due to the suppressive immune microenvironment of solid tumors, the clinical application of TCR-T-cell therapy for EBV-associated solid tumors is limited. Figuring out the mechanism by which EBV participates in the formation of the tumor immunosuppressive microenvironment will help T cells or TCR-T cells break through the limitation and exert stronger antitumor potential.
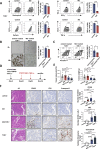
Methods: Flow cytometry was used for analyzing macrophage differentiation phenotypes induced by EBV-infected and EBV-uninfected tumors, as well as the function of T cells co-cultured with these macrophages. Xenograft model in mice was used to explore the effects of M2 macrophages, TCR-T cells, and matrix metalloprotein 9 (MMP9) inhibitors on the growth of EBV-infected tumors.
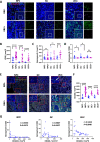
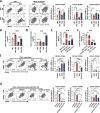
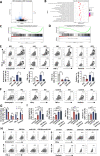
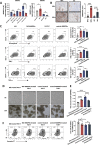
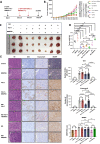
Results: EBV-positive tumors exhibited an exhaustion profile of T cells, despite the presence of a large T-cell infiltration. EBV-infected tumors recruited a large number of mononuclear macrophages with CCL5 and induced CD163+M2 macrophages polarization through the secretion of CSF1 and the promotion of autocrine IL10 production by mononuclear macrophages. Massive secretion of MMP9 by this group of CD163+M2 macrophages induced by EBV infection was an important factor contributing to T-cell exhaustion and TCR-T-cell therapy resistance in EBV-positive tumors, and the use of MMP9 inhibitors improved the function of T cells cocultured with M2 macrophages. Finally, the combination of an MMP9 inhibitor with TCR-T cells targeting EBV-positive tumors significantly inhibited the growth of xenografts in mice.
Conclusions: MMP9 inhibitors improve TCR-T cell function suppressed by EBV-induced M2 macrophages. TCR-T-cell therapy combined with MMP9 inhibitors was an effective therapeutic strategy for EBV-positive solid tumors.
Keywords: Head and Neck Neoplasms; Immune Evation; Immunotherapy; Macrophages; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures

References
-
- Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Fields Virology. 2007.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
