Adiposity in mares induces insulin dysregulation and mitochondrial dysfunction which can be mitigated by nutritional intervention
- PMID: 38886475
- PMCID: PMC11183153
- DOI: 10.1038/s41598-024-64628-x
Adiposity in mares induces insulin dysregulation and mitochondrial dysfunction which can be mitigated by nutritional intervention
Abstract
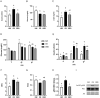
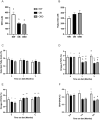
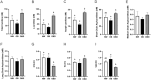
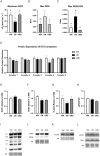
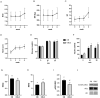
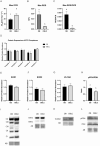
Obesity is a complex disease associated with augmented risk of metabolic disorder development and cellular dysfunction in various species. The goal of the present study was to investigate the impacts of obesity on the metabolic health of old mares as well as test the ability of diet supplementation with either a complex blend of nutrients designed to improve equine metabolism and gastrointestinal health or L-carnitine alone to mitigate negative effects of obesity. Mares (n = 19, 17.9 ± 3.7 years) were placed into one of three group: normal-weight (NW, n = 6), obese (OB, n = 7) or obese fed a complex diet supplement for 12 weeks (OBD, n = 6). After 12 weeks and completion of sample collections, OB mares received L-carnitine alone for an additional 6 weeks. Obesity in mares was significantly associated with insulin dysregulation, reduced muscle mitochondrial function, and decreased skeletal muscle oxidative capacity with greater ROS production when compared to NW. Obese mares fed the complex diet supplement had better insulin sensivity, greater cell lipid metabolism, and higher muscle oxidative capacity with reduced ROS production than OB. L-carnitine supplementation alone did not significantly alter insulin signaling, but improved lipid metabolism and muscle oxidative capacity with reduced ROS. In conclusion, obesity is associated with insulin dysregulation and altered skeletal muscle metabolism in older mares. However, dietary interventions are an effective strategy to improve metabolic status and skeletal muscle mitochondrial function in older mares.
Keywords: Equine; Insulin dysregulation; Metabolism; Mitochondrial dysfunction; Muscle; Obesity; Systemic.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
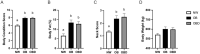

Similar articles
-
Follicular metabolic alterations are associated with obesity in mares and can be mitigated by dietary supplementation.Sci Rep. 2024 Mar 30;14(1):7571. doi: 10.1038/s41598-024-58323-0. Sci Rep. 2024. PMID: 38555310 Free PMC article.
-
Carnitine supplementation in high-fat diet-fed rats does not ameliorate lipid-induced skeletal muscle mitochondrial dysfunction in vivo.Am J Physiol Endocrinol Metab. 2015 Oct 1;309(7):E670-8. doi: 10.1152/ajpendo.00144.2015. Epub 2015 Aug 18. Am J Physiol Endocrinol Metab. 2015. PMID: 26286868
-
Obesity in mares promotes uterine inflammation and alters embryo lipid fingerprints and homeostasis.Biol Reprod. 2018 Oct 1;99(4):761-772. doi: 10.1093/biolre/ioy107. Biol Reprod. 2018. PMID: 29741587
-
Skeletal muscle insulin resistance: role of mitochondria and other ROS sources.J Endocrinol. 2017 Apr;233(1):R15-R42. doi: 10.1530/JOE-16-0598. J Endocrinol. 2017. PMID: 28232636 Review.
-
The manifold role of the mitochondria in skeletal muscle insulin resistance.Curr Opin Clin Nutr Metab Care. 2018 Jul;21(4):267-272. doi: 10.1097/MCO.0000000000000480. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29847447 Free PMC article. Review.
Cited by
-
Nutraceutical Supplement Mitigates Insulin Resistance in Horses with a History of Insulin Dysregulation During a Challenge with a High-Starch Diet.Animals (Basel). 2024 Nov 25;14(23):3385. doi: 10.3390/ani14233385. Animals (Basel). 2024. PMID: 39682351 Free PMC article.
References
-
- Rendle D, et al. Equine obesity: current perspectives. UK-Vet. Equine. 2018;2:1–19. doi: 10.12968/ukve.2018.2.S2.3. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

