Enhancement of erythropoietic output by Cas9-mediated insertion of a natural variant in haematopoietic stem and progenitor cells
- PMID: 38886504
- PMCID: PMC11668683
- DOI: 10.1038/s41551-024-01222-6
Enhancement of erythropoietic output by Cas9-mediated insertion of a natural variant in haematopoietic stem and progenitor cells
Abstract
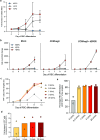
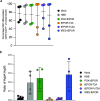
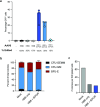
Some gene polymorphisms can lead to monogenic diseases, whereas other polymorphisms may confer beneficial traits. A well-characterized example is congenital erythrocytosis-the non-pathogenic hyper-production of red blood cells-that is caused by a truncated erythropoietin receptor. Here we show that Cas9-mediated genome editing in CD34+ human haematopoietic stem and progenitor cells (HSPCs) can recreate the truncated form of the erythropoietin receptor, leading to substantial increases in erythropoietic output. We also show that combining the expression of the cDNA of a truncated erythropoietin receptor with a previously reported genome-editing strategy to fully replace the HBA1 gene with an HBB transgene in HSPCs (to restore normal haemoglobin production in cells with a β-thalassaemia phenotype) gives the edited HSPCs and the healthy red blood cell phenotype a proliferative advantage. Combining knowledge of human genetics with precise genome editing to insert natural human variants into therapeutic cells may facilitate safer and more effective genome-editing therapies for patients with genetic diseases.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: M.H.P. serves on the scientific advisory board of Allogene Tx and is an advisor to Versant Ventures. M.H.P., M.K.C. and J.C. have equity in Graphite Bio. M.H.P. has equity in CRISPR Tx and serves on the board of directors of Graphite Bio and Kamau Therapeutics. J.C., M.K.C. and M.H.P. have filed provisional patent number PCT/US2022/077951. All other authors declare no competing interests.
Figures


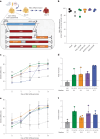
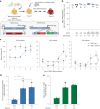
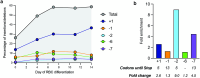

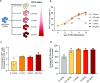


Similar articles
-
Dual α-globin-truncated erythropoietin receptor knockin restores hemoglobin production in α-thalassemia-derived erythroid cells.Cell Rep. 2025 Jan 28;44(1):115141. doi: 10.1016/j.celrep.2024.115141. Epub 2025 Jan 3. Cell Rep. 2025. PMID: 39754719 Free PMC article.
-
Optimization of CRISPR/Cas9 Delivery to Human Hematopoietic Stem and Progenitor Cells for Therapeutic Genomic Rearrangements.Mol Ther. 2019 Jan 2;27(1):137-150. doi: 10.1016/j.ymthe.2018.10.008. Epub 2018 Oct 17. Mol Ther. 2019. PMID: 30424953 Free PMC article.
-
Induction of therapeutic levels of HbF in genome-edited primary β0 39-thalassaemia haematopoietic stem and progenitor cells.Br J Haematol. 2021 Jan;192(2):395-404. doi: 10.1111/bjh.17167. Epub 2020 Nov 20. Br J Haematol. 2021. PMID: 33216968
-
Gene therapy using haematopoietic stem and progenitor cells.Nat Rev Genet. 2021 Apr;22(4):216-234. doi: 10.1038/s41576-020-00298-5. Epub 2020 Dec 10. Nat Rev Genet. 2021. PMID: 33303992 Review.
-
CRISPR/Cas-based gene editing in therapeutic strategies for beta-thalassemia.Hum Genet. 2023 Dec;142(12):1677-1703. doi: 10.1007/s00439-023-02610-9. Epub 2023 Oct 25. Hum Genet. 2023. PMID: 37878144 Review.
Cited by
-
Gene Editing: An Effective Tool for the Future Treatment of Kidney Disease.J Inflamm Res. 2025 Mar 17;18:4001-4018. doi: 10.2147/JIR.S506760. eCollection 2025. J Inflamm Res. 2025. PMID: 40125088 Free PMC article. Review.
-
Dual α-globin-truncated erythropoietin receptor knockin restores hemoglobin production in α-thalassemia-derived erythroid cells.Cell Rep. 2025 Jan 28;44(1):115141. doi: 10.1016/j.celrep.2024.115141. Epub 2025 Jan 3. Cell Rep. 2025. PMID: 39754719 Free PMC article.
-
Engineering synthetic signaling receptors to enable erythropoietin-free erythropoiesis.Nat Commun. 2025 Jan 29;16(1):1140. doi: 10.1038/s41467-025-56239-5. Nat Commun. 2025. PMID: 39880867 Free PMC article.
-
Commentary: Establishment of reference intervals for complete blood count in healthy adults at different altitudes on the Western Sichuan Plateau.Front Med (Lausanne). 2025 Jul 9;12:1642003. doi: 10.3389/fmed.2025.1642003. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40703258 Free PMC article. No abstract available.
-
Introducing a hemoglobin G-Makassar variant in HSCs by in vivo base editing treats sickle cell disease in mice.Mol Ther. 2024 Dec 4;32(12):4353-4371. doi: 10.1016/j.ymthe.2024.10.018. Epub 2024 Oct 28. Mol Ther. 2024. PMID: 39489920 Free PMC article.
References
-
- Hütter, G. et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med.360, 692–698 (2009). - PubMed
-
- Frangoul, H. et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med.384, 252–260 (2021). - PubMed
-
- Sokol, L. et al. Primary familial polycythemia: a frameshift mutation in the erythropoietin receptor gene and increased sensitivity of erythroid progenitors to erythropoietin. Blood86, 15–22 (1995). - PubMed
MeSH terms
Substances
Grants and funding
- Junior Faculty Scholar Award/American Society of Hematology (ASH)
- Minority Medical Student Award/American Society of Hematology (ASH)
- Medical Scientist Training Program/SU | School of Medicine, Stanford University (Stanford School of Medicine, Stanford University)
- Stanford Medical Scholars Research Program/SU | School of Medicine, Stanford University (Stanford School of Medicine, Stanford University)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

