Intravesical instillation-based mTOR-STAT3 dual targeting for bladder cancer treatment
- PMID: 38886756
- PMCID: PMC11184849
- DOI: 10.1186/s13046-024-03088-7
Intravesical instillation-based mTOR-STAT3 dual targeting for bladder cancer treatment
Erratum in
-
Correction: Intravesical instillation-based mTOR-STAT3 dual targeting for bladder cancer treatment.J Exp Clin Cancer Res. 2025 Jul 17;44(1):210. doi: 10.1186/s13046-025-03469-6. J Exp Clin Cancer Res. 2025. PMID: 40676701 Free PMC article. No abstract available.
Abstract
Background: Recent intravesical administration of adenoviral vectors, either as a single injection or in combination with immune checkpoint inhibitors, exemplified by cretostimogene grenadenorepvec and nadofaragene firadenovec, has demonstrated remarkable efficacy in clinical trials for non-muscle invasive bladder cancer. Despite their ability to induce an enhanced immune reaction within the lesion, the intracellular survival signaling of cancer cells has not been thoroughly addressed.
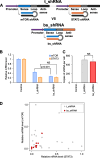
Methods: An analysis of the prognostic data revealed a high probability of therapeutic efficacy with simultaneous inhibition of mTOR and STAT3. Considering the challenges of limited pharmaco-accessibility to the bladder due to its pathophysiological structure and the partially undruggable nature of target molecules, we designed a dual siRNA system targeting both mRNAs. Subsequently, this dual siRNA system was encoded into the adenovirus 5/3 (Ad 5/3) to enhance in vivo delivery efficiency.
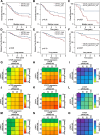
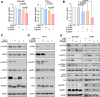
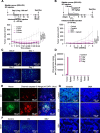
Results: Gene-targeting efficacy was assessed using cells isolated from xenografted tumors using a single-cell analysis system. Our strategy demonstrated a balanced downregulation of mTOR and STAT3 at the single-cell resolution, both in vitro and in vivo. This approach reduced tumor growth in bladder cancer xenograft and orthotopic animal experiments. In addition, increased infiltration of CD8+ T cells was observed in a humanized mouse model. We provided helpful and safe tissue distribution data for intravesical therapy of siRNAs coding adenoviruses.
Conclusions: The bi-specific siRNA strategy, encapsulated in an adenovirus, could be a promising tool to augment cancer treatment efficacy and overcome conventional therapy limitations associated with "undruggability." Hence, we propose that dual targeting of mTOR and STAT3 is an advantageous strategy for intravesical therapy using adenoviruses.
Keywords: Bladder cancer; Cancer therapy; STAT3; mTOR; siRNA.
© 2024. The Author(s).
Conflict of interest statement
D.H.L., J.K.Y., K.H.U., W.H., and M.J.K. have partially received the research grant from Curigin. Ltd. S.M.L., J.P., J.S., and J.W.C. declare no competing interests associated with this manuscript.
Figures





References
-
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–3. - PubMed
-
- Balar AV, Milowsky MI, O’Donnell PH, Alva AS, Kollmeier M, Rose TL, Pitroda S, Kaffenberger SD, Rosenberg JE, Francese K, Hochman T, Goldberg JD, Griglun S, Leis D, Steinberg GD, Wysock J, Schiff PB, Sanfilippo NJ, Taneja S, Huang WC. Pembrolizumab (pembro) in combination with gemcitabine (Gem) and concurrent hypofractionated radiation therapy (RT) as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder (MIBC): A multicenter phase 2 trial. J Clin Oncol. 2021;39((15_suppl)):4504.
-
- Shore ND, Palou RJ, Robert G, Hutson TE, Cesari R, Hariharan S, Rodríguez FÓ, Briganti A, Steinberg GD. Non-muscle-invasive bladder cancer: an overview of potential new treatment options. Urol Oncol. 2021;39(10):642–63. - PubMed
-
- Miodonski AJ, Litwin JA. Microvascular architecture of the human urinary bladder wall: a corrosion casting study. Anat Rec. 1999;254(3):375–81. - PubMed
-
- Uchio EM, Lamm DL, Shore ND, Kamat AM, Tyson M, Tran B, Anderson P, Grandi P, Burke JM. A phase 3, single-arm study of CG0070 in subjects with nonmuscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette-Guerin (BCG). J Clin Oncol. 2022;40(6_suppl):TPS598–TPS598.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

