Defatting of donor transplant livers during normothermic perfusion-a randomised clinical trial: study protocol for the DeFat study
- PMID: 38886851
- PMCID: PMC11181618
- DOI: 10.1186/s13063-024-08189-4
Defatting of donor transplant livers during normothermic perfusion-a randomised clinical trial: study protocol for the DeFat study
Abstract
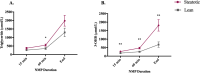
Background: Liver disease is the third leading cause of premature death in the UK. Transplantation is the only successful treatment for end-stage liver disease but is limited by a shortage of suitable donor organs. As a result, up to 20% of patients on liver transplant waiting lists die before receiving a transplant. A third of donated livers are not suitable for transplant, often due to steatosis. Hepatic steatosis, which affects 33% of the UK population, is strongly associated with obesity, an increasing problem in the potential donor pool. We have recently tested defatting interventions during normothermic machine perfusion (NMP) in discarded steatotic human livers that were not transplanted. A combination of therapies including forskolin (NKH477) and L-carnitine to defat liver cells and lipoprotein apheresis filtration were investigated. These interventions resulted in functional improvement during perfusion and reduced the intrahepatocellular triglyceride (IHTG) content. We hypothesise that defatting during NMP will allow more steatotic livers to be transplanted with improved outcomes.
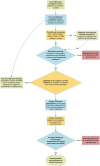
Methods: In the proposed multi-centre clinical trial, we will randomly assign 60 livers from donors with a high-risk of hepatic steatosis to either NMP alone or NMP with defatting interventions. We aim to test the safety and feasibility of the defatting intervention and will explore efficacy by comparing ex-situ and post-reperfusion liver function between the groups. The primary endpoint will be the proportion of livers that achieve predefined functional criteria during perfusion which indicate potential suitability for transplantation. These criteria reflect hepatic metabolism and injury and include lactate clearance, perfusate pH, glucose metabolism, bile composition, vascular flows and transaminase levels. Clinical secondary endpoints will include proportion of livers transplanted in the two arms, graft function; cell-free DNA (cfDNA) at follow-up visits; patient and graft survival; hospital and ITU stay; evidence of ischemia-reperfusion injury (IRI); non-anastomotic biliary strictures and recurrence of steatosis (determined on MRI at 6 months).
Discussion: This study explores ex-situ pharmacological optimisation of steatotic donor livers during NMP. If the intervention proves effective, it will allow the safe transplantation of livers that are currently very likely to be discarded, thereby reducing waiting list deaths.
Trial registration: ISRCTN ISRCTN14957538. Registered in October 2022.
Keywords: Defatting; Functional assessment; Hepatic steatosis; Liver transplantation; Non-alcoholic fatty liver disease (NAFLD); Normothermic machine perfusion (NMP).
© 2024. The Author(s).
Conflict of interest statement
Peter Friend and Constantin Coussios are co-founders and shareholders in OrganOx (a University of Oxford spinout company). They receive consultancy payments as non-executive chief medical and chief technical officers, respectively, of the company.
Simon Knight, David Nasralla and Carlo Ceresa have received consultancy income from OrganOx for assisting with the design and conduct of previous trials. Hynek Mergental is employed by Transmedics who have no involvement or participation in the DeFat study.
Peter Friend, Constantin Coussios and Simon Knight will be not be involved in approaching, consenting, recruiting or in the clinical management of patients in the proposed trial (the Oxford Transplant Centre is not a liver transplant unit).
Figures




References
-
- British Liver Trust. The alarming impact of liver disease in the UK. 2019. Available from: https://britishlivertrust.org.uk/wp-content/uploads/The-alarming-impact-.... Accessed 4 Nov 2019.
-
- British Liver Trust. Becoming a living liver donor. Why do we need more living liver donors? Available from: https://britishlivertrust.org.uk/becoming-a-living-liver-donor. Accessed 22 Nov 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

