Standardized generation of human iPSC-derived hematopoietic organoids and macrophages utilizing a benchtop bioreactor platform under fully defined conditions
- PMID: 38886860
- PMCID: PMC11184717
- DOI: 10.1186/s13287-024-03785-2
Standardized generation of human iPSC-derived hematopoietic organoids and macrophages utilizing a benchtop bioreactor platform under fully defined conditions
Abstract
Background: There is a significant demand for intermediate-scale bioreactors in academic and industrial institutions to produce cells for various applications in drug screening and/or cell therapy. However, the application of these bioreactors in cultivating hiPSC-derived immune cells and other blood cells is noticeably lacking. To address this gap, we have developed a xeno-free and chemically defined intermediate-scale bioreactor platform, which allows for the generation of standardized human iPSC-derived hematopoietic organoids and subsequent continuous production of macrophages (iPSC-Mac).
Methods: We describe a novel method for intermediate-scale immune cell manufacturing, specifically the continuous production of functionally and phenotypically relevant macrophages that are harvested on weekly basis for multiple weeks.
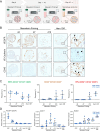
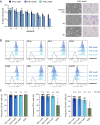
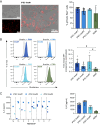
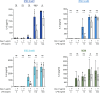
Results: The continuous production of standardized human iPSC-derived macrophages (iPSC-Mac) from 3D hematopoietic organoids also termed hemanoids, is demonstrated. The hemanoids exhibit successive stage-specific embryonic development, recapitulating embryonic hematopoiesis. iPSC-Mac were efficiently and continuously produced from three different iPSC lines and exhibited a consistent and reproducible phenotype, as well as classical functionality and the ability to adapt towards pro- and anti-inflammatory activation stages. Single-cell transcriptomic analysis revealed high macrophage purity. Additionally, we show the ability to use the produced iPSC-Mac as a model for testing immunomodulatory drugs, exemplified by dexamethasone.
Conclusions: The novel method demonstrates an easy-to-use intermediate-scale bioreactor platform that produces prime macrophages from human iPSCs. These macrophages are functionally active and require no downstream maturation steps, rendering them highly desirable for both therapeutic and non-therapeutic applications.
Keywords: Bioreactor; Cell manufacturing; Drug screening; Hematopoiesis; Macrophages; Organoids; Up-scaling; hiPSC.
© 2024. The Author(s).
Conflict of interest statement
M.A. and N.L. are authors of the patent application (European patent application number PCT/EP2018/061574) entitled ‘Stem-cell derived myeloid cells, generation and use thereof’. The priority date of the application is 4 May 2017. N.L. and S.A. are authors on the patent application (European patent application number PCT/EP2021/083371) entitled ‘Application of stem cell derived monocytes in a Monocyte Activation Test (MAT) for the assessment of pyrogenicity and inflammatory potential’. The priority date of the application is 29 November 2021. All other authors declare no competing interests.
Figures


References
-
- Saleh F, Mondeh-Lowor R, de Peppo GM. Xeno-free cultivation of human induced pluripotent stem cells for clinical applications. Methods in iPSC Technology. Elsevier; 2021. p. 309–41.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

