Effects of cerebellar repetitive transcranial magnetic stimulation plus physiotherapy in spinocerebellar ataxias - A randomized clinical trial
- PMID: 38887169
- PMCID: PMC11183922
- DOI: 10.1111/cns.14797
Effects of cerebellar repetitive transcranial magnetic stimulation plus physiotherapy in spinocerebellar ataxias - A randomized clinical trial
Abstract
Background: In absence of drug therapy options, standard treatment for spinocerebellar ataxia consists of symptomatic physiotherapy and speech therapy. New therapeutic options are urgently needed. Transcranial magnetic stimulation is a promising therapeutic option, but applicability is limited by lengthy duration of stimulation protocols.
Methods: In this randomized sham controlled clinical trial, patients were assigned to verum (n = 15) or sham (n = 18) cerebellar transcranial magnetic stimulation. To yield best possible treatment effects, both intervention groups received intensified physiotherapy for the duration of the study.
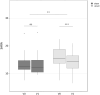
Results: Ataxia severity was reduced by 1.6 points on the Scale for assessment and Rating of Ataxia among patients in the verum group (p < 0.001). Clinical improvement was significantly larger in the verum group, compared to the sham group (p < 0.01). The treatment effect was mainly carried by improved appendicular coordination. Patients in the verum group also significantly improved in the 8 Meter Walk Test (p < 0.05) and PATA rate (p < 0.01).
Conclusions: Cerebellar rTMS ameliorates ataxia severity in patient with spinocerebellar ataxia. Condensing treatment duration to only 5 days without reduction of treatment effects facilitates applicability and therefore broadens availability to larger patient populations.
Keywords: SCA; TMS; ataxia; cerebellum; transcranial magnetic stimulation.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
MGE received research support from the German Ministry of Education and Research (BMBF) within the European Joint Program for Rare Diseases (EJP‐RD) 2021 Transnational Call for Rare Disease Research Projects (funding number 01GM2110), from the National Ataxia Foundation (NAF), and from Ataxia UK, and received consulting fees from Healthcare Manufaktur, Germany, all unrelated to this study. MGE is member of the European Reference Network for Rare Neurological Diseases (ERN‐RD). AF received travel support from CSL Behring, Ipsen, Ever Pharma and Indorsia, all unrelated to this project. AF and OK received research support from the German Parkinson's Association (Deutsche Parkinson Vereinigung), unrelated to this project.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources