GIP attenuates neuronal oxidative stress by regulating glucose uptake in spinal cord injury of rat
- PMID: 38887182
- PMCID: PMC11183929
- DOI: 10.1111/cns.14806
GIP attenuates neuronal oxidative stress by regulating glucose uptake in spinal cord injury of rat
Abstract
Aim: Glucose-dependent insulinotropic polypeptide (GIP) is a ligand of glucose-dependent insulinotropic polypeptide receptor (GIPR) that plays an important role in the digestive system. In recent years, GIP has been regarded as a hormone-like peptide to regulate the local metabolic environment. In this study, we investigated the antioxidant role of GIP on the neuron and explored the possible mechanism.
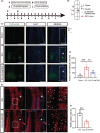
Methods: Cell counting Kit-8 (CCK-8) was used to measure cell survival. TdT-mediated dUTP Nick-End Labeling (TUNEL) was used to detect apoptosis in vitro and in vivo. Reactive oxygen species (ROS) levels were probed with 2', 7'-Dichloro dihydrofluorescein diacetate (DCFH-DA), and glucose intake was detected with 2-NBDG. Immunofluorescence staining and western blot were used to evaluate the protein level in cells and tissues. Hematoxylin-eosin (HE) staining, immunofluorescence staining and tract-tracing were used to observe the morphology of the injured spinal cord. Basso-Beattie-Bresnahan (BBB) assay was used to evaluate functional recovery after spinal cord injury.
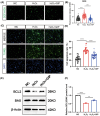
Results: GIP reduced the ROS level and protected cells from apoptosis in cultured neurons and injured spinal cord. GIP facilitated wound healing and functional recovery of the injured spinal cord. GIP significantly improved the glucose uptake of cultured neurons. Meanwhile, inhibition of glucose uptake significantly attenuated the antioxidant effect of GIP. GIP increased glucose transporter 3 (GLUT3) expression via up-regulating the level of hypoxia-inducible factor 1α (HIF-1α) in an Akt-dependent manner.
Conclusion: GIP increases GLUT3 expression and promotes glucose intake in neurons, which exerts an antioxidant effect and protects neuronal cells from oxidative stress both in vitro and in vivo.
Keywords: Akt; GIP; HIF‐1α; glucose transporter; oxidative stress; spinal cord injury.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declared that they have no conflicts of interest with the contents of this article.
Figures






References
-
- Gasbjerg LS, Gabe MBN, Hartmann B, et al. Glucose‐dependent insulinotropic polypeptide (GIP) receptor antagonists as anti‐diabetic agents. Peptides. 2018;100:173‐181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- KYCX23_3409/Postgraduate Research & Practice Innovation Program of Jiangsu Province
- 21KJB180010/Natural Science Research of Jiangsu Higher Education Institutions of China
- 31970412/National Natural Science Foundation of China
- 32070725/National Natural Science Foundation of China
- 82101464/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

