Polyoxometalate Clusters Confined in Reduced Graphene Oxide Membranes for Effective Ion Sieving and Desalination
- PMID: 38887207
- PMCID: PMC11422814
- DOI: 10.1002/advs.202402018
Polyoxometalate Clusters Confined in Reduced Graphene Oxide Membranes for Effective Ion Sieving and Desalination
Abstract
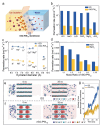
Efficient 2D membranes play a critical role in water purification and desalination. However, most 2D membranes, such as graphene oxide (GO) membranes, tend to swell or disintegrate in liquid, making precise ionic sieving a tough challenge. Herein, the fabrication of the polyoxometalate clusters (PW12) intercalated reduced graphene oxide (rGO) membrane (rGO-PW12) is reported through a polyoxometalate-assisted in situ photoreduction strategy. The intercalated PW12 result in the interlayer spacing in the sub-nanometer scale and induce a nanoconfinement effect to repel the ions in various salt solutions. The permeation rate of rGO-PW12 membranes are about two orders of magnitude lower than those through the GO membrane. The confinement of nanochannels also generate the excellent non-swelling stability of rGO-PW12 membranes in aqueous solutions up to 400 h. Moreover, when applied in forward osmosis, the rGO-PW12 membranes with a thickness of 90 nm not only exhibit a high-water permeance of up to 0.11790 L m-2 h-1 bar-1 and high NaCl rejection (98.3%), but also reveal an ultrahigh water/salt selectivity of 4740. Such significantly improved ion-exclusion ability and high-water flux benefit from the multi-interactions and nanoconfinement effect between PW12 and rGO nanosheets, which afford a well-interlinked lamellar structure via hydrogen bonding and van der Waals interactions.
Keywords: desalination; ion sieving; membranes; polyoxometalates; reduced graphene oxide.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Lim Y. J., Goh K., Kurihara M., Wang R., J. Membrane. Sci. 2021, 629, 119292;
- b) Klaysom C., Cath T. Y., Depuydt T., Vankelecom I. F. J., Chem. Soc. Rev. 2013, 42, 6959; - PubMed
- c) Mauter M. S., Zucker I., Perreault F., Werber J. R., Kim J.‐H., Elimelech M., Nat. Sustain. 2018, 1, 166.
-
- a) Wang J., Zhou H., Li S., Wang L., Angew. Chem., Int. Ed. 2023, 62, e202218321; - PubMed
- b) Guo J., Zhang Y., Yang F., Mamba B. B., Ma J., Shao L., Liu S., Angew. Chem., Int. Ed. 2023, 62, e202302931; - PubMed
- c) Zhan H., Xiong Z., Cheng C., Liang Q., Liu J. Z., Li D., Adv. Mater. 2020, 32, 1904562. - PubMed
-
- a) Shen J., Liu G., Han Y., Jin W., Nat. Rev. Mater. 2021, 6, 294;
- b) Wang L., Boutilier M. S. H., Kidambi P. R., Jang D., Hadjiconstantinou N. G., Karnik R., Nat. Nanotechnol. 2017, 12, 509; - PubMed
- c) Kang Y., Xia Y., Wang H., Zhang X., Adv. Funct. Mater. 2019, 29, 1902014;
- d) Kang Y., Hu T., Wang Y., He K., Wang Z., Hora Y., Zhao W., Xu R., Chen Y., Xie Z., Wang H., Gu Q., Zhang X., Nat. Commun. 2023, 14, 4075. - PMC - PubMed
-
- Surwade S. P., Smirnov S. N., Vlassiouk I. V., Unocic R. R., Veith G. M., Dai S., Mahurin S. M., Nat. Nanotechnol. 2016, 11, 995. - PubMed
-
- Liu G., Jin W., Xu N., Chem. Soc. Rev. 2015, 44, 5016. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
