Lateral parabrachial nucleus astrocytes control food intake
- PMID: 38887265
- PMCID: PMC11180714
- DOI: 10.3389/fendo.2024.1389589
Lateral parabrachial nucleus astrocytes control food intake
Abstract
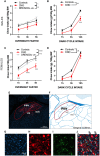
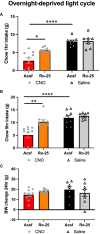
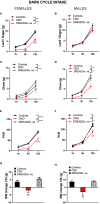
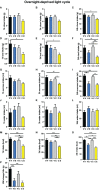
Food intake behavior is under the tight control of the central nervous system. Most studies to date focus on the contribution of neurons to this behavior. However, although previously overlooked, astrocytes have recently been implicated to play a key role in feeding control. Most of the recent literature has focused on astrocytic contribution in the hypothalamus or the dorsal vagal complex. The contribution of astrocytes located in the lateral parabrachial nucleus (lPBN) to feeding behavior control remains poorly understood. Thus, here, we first investigated whether activation of lPBN astrocytes affects feeding behavior in male and female rats using chemogenetic activation. Astrocytic activation in the lPBN led to profound anorexia in both sexes, under both ad-libitum feeding schedule and after a fasting challenge. Astrocytes have a key contribution to glutamate homeostasis and can themselves release glutamate. Moreover, lPBN glutamate signaling is a key contributor to potent anorexia, which can be induced by lPBN activation. Thus, here, we determined whether glutamate signaling is necessary for lPBN astrocyte activation-induced anorexia, and found that pharmacological N-methyl D-aspartate (NMDA) receptor blockade attenuated the food intake reduction resulting from lPBN astrocyte activation. Since astrocytes have been shown to contribute to feeding control by modulating the feeding effect of peripheral feeding signals, we further investigated whether lPBN astrocyte activation is capable of modulating the anorexic effect of the gut/brain hormone, glucagon like peptide -1, as well as the orexigenic effect of the stomach hormone - ghrelin, and found that the feeding effect of both signals is modulated by lPBN astrocytic activation. Lastly, we found that lPBN astrocyte activation-induced anorexia is affected by a diet-induced obesity challenge, in a sex-divergent manner. Collectively, current findings uncover a novel role for lPBN astrocytes in feeding behavior control.
Keywords: GLP-1; NMDA; astrocytes; ghrelin; glia; high-fat diet; hindbrain; lateral parabrachial nucleus.
Copyright © 2024 Mishra, Richard, Maric, Shevchouk, Börchers, Eerola, Krieger and Skibicka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
GLP-1 receptor stimulation of the lateral parabrachial nucleus reduces food intake: neuroanatomical, electrophysiological, and behavioral evidence.Endocrinology. 2014 Nov;155(11):4356-67. doi: 10.1210/en.2014-1248. Epub 2014 Aug 13. Endocrinology. 2014. PMID: 25116706 Free PMC article.
-
Glucagon-like Peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed.Neuropsychopharmacology. 2014 Aug;39(9):2233-43. doi: 10.1038/npp.2014.74. Epub 2014 Mar 26. Neuropsychopharmacology. 2014. PMID: 24681814 Free PMC article.
-
Peptide YY signaling in the lateral parabrachial nucleus increases food intake through the Y1 receptor.Am J Physiol Endocrinol Metab. 2015 Oct 15;309(8):E759-66. doi: 10.1152/ajpendo.00346.2015. Epub 2015 Sep 1. Am J Physiol Endocrinol Metab. 2015. PMID: 26330345 Free PMC article.
-
Role of α2-adrenoceptors in the lateral parabrachial nucleus in the control of body fluid homeostasis.Braz J Med Biol Res. 2014 Jan;47(1):11-8. doi: 10.1590/1414-431X20133308. Epub 2014 Jan 10. Braz J Med Biol Res. 2014. PMID: 24519089 Free PMC article. Review.
-
Astrocytes of the hippocampus and responses to periprandial neuroendocrine hormones.Physiol Behav. 2025 Jun 1;295:114913. doi: 10.1016/j.physbeh.2025.114913. Epub 2025 Apr 8. Physiol Behav. 2025. PMID: 40209869 Review.
Cited by
-
Caveolae with GLP-1 and NMDA Receptors as Crossfire Points for the Innovative Treatment of Cognitive Dysfunction Associated with Neurodegenerative Diseases.Molecules. 2024 Aug 20;29(16):3922. doi: 10.3390/molecules29163922. Molecules. 2024. PMID: 39203005 Free PMC article. Review.
-
Astrocyte involvement in metabolic regulation and disease.Trends Endocrinol Metab. 2025 Mar;36(3):219-234. doi: 10.1016/j.tem.2024.08.001. Epub 2024 Aug 29. Trends Endocrinol Metab. 2025. PMID: 39214743 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

