A local drug delivery system prolongs graft survival by dampening T cell infiltration and neutrophil extracellular trap formation in vascularized composite allografts
- PMID: 38887281
- PMCID: PMC11180892
- DOI: 10.3389/fimmu.2024.1387945
A local drug delivery system prolongs graft survival by dampening T cell infiltration and neutrophil extracellular trap formation in vascularized composite allografts
Abstract
Introduction: The standard treatment for preventing rejection in vascularized composite allotransplantation (VCA) currently relies on systemic immunosuppression, which exposes the host to well-known side effects. Locally administered immunosuppression strategies have shown promising results to bypass this hurdle. Nevertheless, their progress has been slow, partially attributed to a limited understanding of the essential mechanisms underlying graft rejection. Recent discoveries highlight the crucial involvement of innate immune components, such as neutrophil extracellular traps (NETs), in organ transplantation. Here we aimed to prolong graft survival through a tacrolimus-based drug delivery system and to understand the role of NETs in VCA graft rejection.
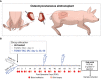
Methods: To prevent off-target toxicity and promote graft survival, we tested a locally administered tacrolimus-loaded on-demand drug delivery system (TGMS-TAC) in a multiple MHC-mismatched porcine VCA model. Off-target toxicity was assessed in tissue and blood. Graft rejection was evaluated macroscopically while the complement system, T cells, neutrophils and NETs were analyzed in graft tissues by immunofluorescence and/or western blot. Plasmatic levels of inflammatory cytokines were measured using a Luminex magnetic-bead porcine panel, and NETs were measured in plasma and tissue using DNA-MPO ELISA. Lastly, to evaluate the effect of tacrolimus on NET formation, NETs were induced in-vitro in porcine and human peripheral neutrophils following incubation with tacrolimus.
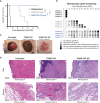
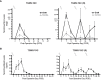
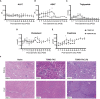
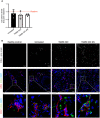
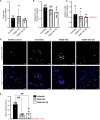
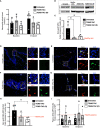
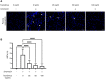
Results: Repeated intra-graft administrations of TGMS-TAC minimized systemic toxicity and prolonged graft survival. Nevertheless, signs of rejection were observed at endpoint. Systemically, there were no increases in cytokine levels, complement anaphylatoxins, T-cell subpopulations, or neutrophils during rejection. Yet, tissue analysis showed local infiltration of T cells and neutrophils, together with neutrophil extracellular traps (NETs) in rejected grafts. Interestingly, intra-graft administration of tacrolimus contributed to a reduction in both T-cellular infiltration and NETs. In fact, in-vitro NETosis assessment showed a 62-84% reduction in NETs after stimulated neutrophils were treated with tacrolimus.
Conclusion: Our data indicate that the proposed local delivery of immunosuppression avoids off-target toxicity while prolonging graft survival in a multiple MHC-mismatch VCA model. Furthermore, NETs are found to play a role in graft rejection and could therefore be a potential innovative therapeutic target.
Keywords: calcineurin inhibitors (CNIs); drug delivery systems (DDSs); local immunosuppression; neutrophil extracellular traps (NETs); porcine model; tacrolimus; transplantation immunology; vascularized composite allotransplantation (VCA).
Copyright © 2024 Arenas Hoyos, Helmer, Yerly, Lese, Hirsiger, Zhang, Casoni, Garcia, Petrucci, Hammer, Duckova, Banz, Montani, Constantinescu, Vögelin, Bordon, Aleandri, Prost, Taddeo, Luciani, Rieben, Sorvillo and Olariu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Gilbert R. Transplant is successful with a cadaver forearm. Med Trib Med News. (1964) 5:20–2.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

