Preparation of an injectable zinc-containing hydrogel with double dynamic bond and its potential application in the treatment of periodontitis
- PMID: 38887645
- PMCID: PMC11181151
- DOI: 10.1039/d4ra00546e
Preparation of an injectable zinc-containing hydrogel with double dynamic bond and its potential application in the treatment of periodontitis
Abstract
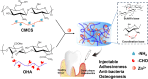
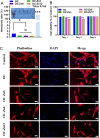
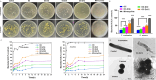
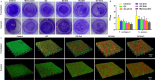
Periodontal tissue regeneration continues to face significant clinical challenges. Periodontitis leads to alveolar bone resorption and even tooth loss due to persistent microbial infection and persistent inflammatory response. As a promising topical drug delivery system, the application of hydrogels in the controlled release of periodontal bioactive drugs has aroused great interest. Therefore, the design and preparation of an injectable hydrogel with self-repairing properties for periodontitis treatment is still in great demand. In this study, polysaccharide-based self-healing hydrogels with antimicrobial osteogenic properties were developed. Zinc ions are introduced into a dynamic cross-linking network formed by dynamic Schiff bases between carboxymethyl chitosan and oxidized hyaluronic acid via coordination bonds. The OC-Zn hydrogels exhibited good tissue adhesion, good fatigue resistance, excellent self-healing ability, low cytotoxicity, good broad-spectrum antimicrobial activity, and osteogenic activity. Therefore, the designed hydrogels allow the development of drug delivery systems as a potential treatment for periodontitis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Smart injectable hydrogels for periodontal regeneration: Recent advancements in biomaterials and biofabrication strategies.Mater Today Bio. 2025 May 11;32:101855. doi: 10.1016/j.mtbio.2025.101855. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40487163 Free PMC article. Review.
-
Injectable and Self-Healing Hydrogels with Double-Dynamic Bond Tunable Mechanical, Gel-Sol Transition and Drug Delivery Properties for Promoting Periodontium Regeneration in Periodontitis.ACS Appl Mater Interfaces. 2021 Dec 29;13(51):61638-61652. doi: 10.1021/acsami.1c18701. Epub 2021 Dec 15. ACS Appl Mater Interfaces. 2021. PMID: 34908393
-
Injectable multifunctional carboxymethyl chitosan/hyaluronic acid hydrogel for drug delivery systems.Int J Biol Macromol. 2023 Sep 30;249:125801. doi: 10.1016/j.ijbiomac.2023.125801. Epub 2023 Jul 11. Int J Biol Macromol. 2023. PMID: 37442509
-
A self-healing, magnetic and injectable biopolymer hydrogel generated by dual cross-linking for drug delivery and bone repair.Acta Biomater. 2022 Nov;153:159-177. doi: 10.1016/j.actbio.2022.09.036. Epub 2022 Sep 22. Acta Biomater. 2022. PMID: 36152907
-
Advances in Injectable and Self-healing Polysaccharide Hydrogel Based on the Schiff Base Reaction.Macromol Rapid Commun. 2021 May;42(10):e2100025. doi: 10.1002/marc.202100025. Epub 2021 Apr 20. Macromol Rapid Commun. 2021. PMID: 33876841 Review.
Cited by
-
Herbal remedies for oral and dental health: a comprehensive review of their multifaceted mechanisms including antimicrobial, anti-inflammatory, and antioxidant pathways.Inflammopharmacology. 2025 Mar;33(3):1085-1160. doi: 10.1007/s10787-024-01631-8. Epub 2025 Feb 5. Inflammopharmacology. 2025. PMID: 39907951 Free PMC article. Review.
-
Smart injectable hydrogels for periodontal regeneration: Recent advancements in biomaterials and biofabrication strategies.Mater Today Bio. 2025 May 11;32:101855. doi: 10.1016/j.mtbio.2025.101855. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40487163 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

