Biomarkers of peanut allergy in children over time
- PMID: 38887787
- PMCID: PMC11875691
- DOI: 10.1111/all.16193
Biomarkers of peanut allergy in children over time
Abstract
Background: Various biomarkers are used to define peanut allergy (PA). We aimed to observe changes in PA resolution and persistence over time comparing biomarkers in PA and peanut sensitised but tolerant (PS) children in a population-based cohort.
Methods: Participants were recruited from the EAT and EAT-On studies, conducted across England and Wales, and were exclusively breastfeed babies recruited at 3 months old and followed up until 7-12 years old. Clinical characteristics, skin prick test (SPT), sIgE to peanut and peanut components and mast cell activation tests (MAT) were assessed at 12 months, 36 months and 7-12 years. PA status was determined at the 7-12 year time point.
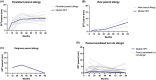
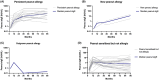
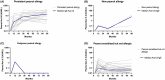
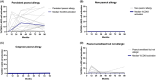
Results: The prevalence of PA was 2.1% at 7-12 years. Between 3 and 7-12 year, two children developed PA and one outgrew PA. PA children had larger SPT, higher peanut-sIgE, Ara h 2-sIgE and MAT (all p < .001) compared to PS children from 12 months onwards. SPT, peanut-sIgE, Ara h 2-sIgE and MAT between children with persistent PA, new PA, outgrown PA and PS were statistically significant from 12 months onwards (p < .001). Those with persistent PA had SPT, peanut-sIgE and Ara h 2-sIgE that increased over time and MAT which was highest at 36 months. New PA children had increased SPT and peanut-sIgE from 36 months to 7-12 years, but MAT remained low. PS children had low biomarkers across time.
Conclusions: In this cohort, few children outgrow or develop new PA between 36 months and 7-12 years. Children with persistent PA have raised SPT, peanut-sIgE, Ara h 2-sIgE and MAT evident from infancy that consistently increase over time.
Keywords: biomarkers; food allergy; mast cell activation test; peanut allergy; tolerance.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Figures
References
-
- Greenhawt M, Shaker M, Wang J, et al. Peanut allergy diagnosis: a 2020 practice parameter update, systematic review, and GRADE analysis. J Allergy Clin Immunol. 2020;146(6):1302‐1334. - PubMed
-
- Peters RL, Allen KJ, Dharmage SC, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: a population‐based assessment. J Allergy Clin Immunol. 2015;135(5):1257‐1266. - PubMed
-
- Beck C, Koplin J, Dharmage S, et al. Persistent food allergy and food allergy coexistent with eczema is associated with reduced growth in the first 4 years of life. J Allergy Clin Immunol Pract. 2015;4:248‐256.e3. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

