Lung-Specific mRNA Delivery Enabled by Sulfonium Lipid Nanoparticles
- PMID: 38888232
- PMCID: PMC12013526
- DOI: 10.1021/acs.nanolett.4c01854
Lung-Specific mRNA Delivery Enabled by Sulfonium Lipid Nanoparticles
Abstract
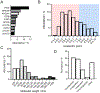
Among various mRNA carrier systems, lipid nanoparticles (LNPs) stand out as the most clinically advanced. While current clinical trials of mRNA/LNP therapeutics mainly address liver diseases, the potential of mRNA therapy extends far beyond─yet to be unraveled. To fully unlock the promises of mRNA therapy, there is an urgent need to develop safe and effective LNP systems that can target extrahepatic organs. Here, we report on the development of sulfonium lipid nanoparticles (sLNPs) for systemic mRNA delivery to the lungs. sLNP effectively and specifically delivered mRNA to the lungs following intravenous administration in mice. No evidence of lung and systemic inflammation or toxicity in major organs was induced by sLNP. Our findings demonstrated that the newly developed lung-specific sLNP platform is both safe and efficacious. It holds great promise for advancing the development of new mRNA-based therapies for the treatment of lung-associated diseases and conditions.
Keywords: Sulfonium lipid nanoparticle; genome engineering; lung targeting; mRNA delivery; pulmonary endothelium.
Conflict of interest statement
Conflict of Interest
A patent application for the lipid materials developed in this study has been filed by the State University of New York.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

