Nanobody-Engineered Biohybrid Bacteria Targeting Gastrointestinal Cancers Induce Robust STING-Mediated Anti-Tumor Immunity
- PMID: 38888519
- PMCID: PMC11336900
- DOI: 10.1002/advs.202401905
Nanobody-Engineered Biohybrid Bacteria Targeting Gastrointestinal Cancers Induce Robust STING-Mediated Anti-Tumor Immunity
Abstract
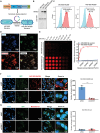
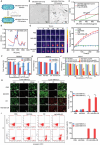
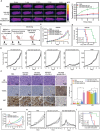
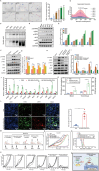
Bacteria can be utilized for cancer therapy owing to their preferential colonization at tumor sites. However, unmodified non-pathogenic bacteria carry potential risks due to their non-specific targeting effects, and their anti-tumor activity is limited when used as monotherapy. In this study, a biohybrid-engineered bacterial system comprising non-pathogenic MG1655 bacteria modified with CDH17 nanobodies on their surface and conjugated with photosensitizer croconium (CR) molecules is developed. The resultant biohybrid bacteria can efficiently home to CDH17-positive tumors, including gastric, pancreatic, and colorectal cancers, and significantly suppress tumor growth upon irradiation. More importantly, biohybrid bacteria-mediated photothermal therapy (PTT) induced abundant macrophage infiltration in a syngeneic murine colorectal model. Further, that the STING pathway is activated in tumor macrophages by the released bacterial nucleic acid after PTT is revealed, leading to the production of type I interferons. The addition of CD47 nanobody but not PD-1 antibody to the PTT regimen can eradicate the tumors and extend survival. This results indicate that bacteria endowed with tumor-specific selectivity and coupled with photothermal payloads can serve as an innovative strategy for low-immunogenicity cancers. This strategy can potentially reprogram the tumor microenvironment by inducing macrophage infiltration and enhancing the efficacy of immunotherapy targeting macrophages.
Keywords: STING; bacteria; immunotherapy; nanobody; photothermal therapy.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Nanobody Engineered and Photosensitiser Loaded Bacterial Outer Membrane Vesicles Potentiate Antitumour Immunity and Immunotherapy.J Extracell Vesicles. 2025 Apr;14(4):e70069. doi: 10.1002/jev2.70069. J Extracell Vesicles. 2025. PMID: 40240911 Free PMC article.
-
Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV+ oral cancer.J Immunother Cancer. 2019 Sep 18;7(1):252. doi: 10.1186/s40425-019-0728-4. J Immunother Cancer. 2019. PMID: 31533840 Free PMC article.
-
A nanobody-guided multifunctional T cell engager promotes strong anti-tumor responses via synergistic immuno-photothermal effects.J Nanobiotechnology. 2024 Sep 14;22(1):561. doi: 10.1186/s12951-024-02781-1. J Nanobiotechnology. 2024. PMID: 39272205 Free PMC article.
-
Quercetin-ferrum nanoparticles enhance photothermal therapy by modulating the tumor immunosuppressive microenvironment.Acta Biomater. 2022 Dec;154:454-466. doi: 10.1016/j.actbio.2022.10.008. Epub 2022 Oct 12. Acta Biomater. 2022. PMID: 36243377
-
The Rational Engineered Bacteria Based Biohybrid Living System for Tumor Therapy.Adv Healthc Mater. 2024 Nov;13(28):e2401538. doi: 10.1002/adhm.202401538. Epub 2024 Jul 25. Adv Healthc Mater. 2024. PMID: 39051784 Review.
Cited by
-
Nanobody Engineered and Photosensitiser Loaded Bacterial Outer Membrane Vesicles Potentiate Antitumour Immunity and Immunotherapy.J Extracell Vesicles. 2025 Apr;14(4):e70069. doi: 10.1002/jev2.70069. J Extracell Vesicles. 2025. PMID: 40240911 Free PMC article.
-
Tumor-derived exosomal CCT6A serves as a matchmaker introducing chemokines to tumor-associated macrophages in pancreatic ductal adenocarcinoma.Cell Death Dis. 2025 May 15;16(1):382. doi: 10.1038/s41419-025-07720-y. Cell Death Dis. 2025. PMID: 40374617 Free PMC article.
-
Enhancing photothermal therapy of tumors with image-guided thermal control of gene-expressing bacteria.Theranostics. 2024 Sep 9;14(15):5945-5964. doi: 10.7150/thno.98257. eCollection 2024. Theranostics. 2024. PMID: 39346533 Free PMC article.
References
-
- a) Chowdhury S., Castro S., Coker C., Hinchliffe T. E., Arpaia N., Danino T., Nat. Med. 2019, 25, 1057; - PMC - PubMed
- b) Gurbatri C. R., Lia I., Vincent R., Coker C., Castro S., Treuting P. M., Hinchliffe T. E., Arpaia N., Danino T., Sci. Transl. Med. 2020, 12, eaax0876; - PMC - PubMed
- c) Zheng J. H., Nguyen V. H., Jiang S. N., Park S. H., Tan W., Hong S. H., Shin M. G., Chung I. J., Hong Y., Bom H. S., Choy H. E., Lee S. E., Rhee J. H., Min J. J., Sci. Transl. Med. 2017, 9, eaak9537. - PubMed
MeSH terms
Substances
Grants and funding
- CI2023D003/Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences
- CI2021B014/Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences
- JCYJ20210324115800001/Science and Technology Foundation of Shenzhen
- JCYJ20210324113608023/Science and Technology Foundation of Shenzhen
- JCYJ20200109120205924/Science and Technology Foundation of Shenzhen
- 2022YFC2303600/National Key Research and Development Program of China
- 2020YFA0908000/National Key Research and Development Program of China
- ZYYCXTD-C-202002/Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine
- CI2023E002/CACMS Innovation Fund
- CI2021A05101/CACMS Innovation Fund
- CI2021A05104/CACMS Innovation Fund
- B2302051/Shenzhen Medical Research Fund
- CW202002/Distinguished Expert Project of Sichuan Province Tianfu Scholar
- ZZ16-ND-10-23/Fundamental Research Funds for the Central public welfare research institutes
- ZZ15-ND-10/Fundamental Research Funds for the Central public welfare research institutes
- ZZ14-ND-010/Fundamental Research Funds for the Central public welfare research institutes
- ZZ14-FL-002/Fundamental Research Funds for the Central public welfare research institutes
- ZZ14-YQ-050/Fundamental Research Funds for the Central public welfare research institutes
- ZZ14-YQ-051/Fundamental Research Funds for the Central public welfare research institutes
- JCYJ20200109120205924/Shenzhen Science and Technology Innovation Commission
- 2021A1515012164/GuangDong Basic and Applied Basic Research Foundation
- 2022A1515110745/GuangDong Basic and Applied Basic Research Foundation
- 2021A1515111188/GuangDong Basic and Applied Basic Research Foundation
- RCYX20221008092950121/Shenzhen Science and Technology Innovation Committee (SZSTI)
- 82373775/National Natural Science Foundation of China
- 82070517/National Natural Science Foundation of China
- 82170842/National Natural Science Foundation of China
- 8230130506/National Natural Science Foundation of China
- 82371572/National Natural Science Foundation of China
- GJHZ20200731095411034/HUILAN Public Welfare, International Science and Technology Cooperation for Shenzhen Technology Innovation Plan
- SYWGSCGZH202304/Shenzhen People's Hospital
- 2022M711328/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Research Materials
