Improved efficacy and safety of zanubrutinib versus ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) in China: a subgroup of ALPINE
- PMID: 38888616
- PMCID: PMC11512864
- DOI: 10.1007/s00277-024-05823-8
Improved efficacy and safety of zanubrutinib versus ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) in China: a subgroup of ALPINE
Abstract
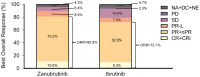
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) has different epidemiology in Chinese vs. Western patients, but there are few studies of CLL/SLL in large populations of Chinese patients. ALPINE is a global phase 3 trial investigating Bruton tyrosine kinase inhibitors zanubrutinib vs. ibrutinib to treat relapsed/refractory (R/R) CLL/SLL. Here we report results from the subgroup of Chinese patients. Adults with R/R CLL/SLL were randomized 1:1 to receive zanubrutinib (160 mg twice-daily) or ibrutinib (420 mg once-daily) until disease progression or unacceptable toxicity. Endpoints included overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and safety. Data were analyzed descriptively. Ninety patients were randomized in China (zanubrutinib, n = 47; ibrutinib, n = 43). Baseline characteristics were balanced between groups, with fewer male patients in the zanubrutinib vs. ibrutinib group (55.3% vs. 69.8%). Median age was 60.5 years, 11% had del(17p) mutation, and 32% had tumor protein 53 (TP53) mutation. With median 25.3 months follow-up, ORR was 80.9% with zanubrutinib vs. 72.1% with ibrutinib. PFS was improved with zanubrutinib vs. ibrutinib (HR = 0.34 [95% CI, 0.15, 0.77]), and the HR for OS was 0.45 (95% CI, 0.14, 1.50). Rates of Grade ≥ 3 treatment-emergent adverse events (TEAEs; 64.4% vs. 72.1%), AEs leading to discontinuation (6.4% vs. 14.0%), and serious TEAEs (35.6% vs. 51.2%) were lower with zanubrutinib vs. ibrutinib. Zanubrutinib demonstrated improved ORR, PFS, and OS vs. ibrutinib and a more favorable safety profile in patients with R/R CLL/SLL in China. These results are consistent with the full global population of ALPINE. ClinicalTrials.gov: NCT03734016, registered November 7, 2018.
Keywords: Chinese population; Chronic lymphocytic leukemia; Ibrutinib; Small lymphocytic lymphoma; Zanubrutinib.
© 2024. The Author(s).
Conflict of interest statement
L.F., L.W., T.S., and K.W. are employed by BeiGene and may hold company stock/stock options. K.Z., T.W., L.P., W.X., J.J., W.Z., Y.H., J.H., R.F., P.L., Z.L., P.L., H.J., S.G., H.Z., K.Y., Z.W., X.Z., Z.S., F.L., D.Y., and J.W. have nothing to disclose. L.Q. participates in speaker’s bureaus with BeiGene, Janssen, Roche, AstraZeneca, and Takeda.
Figures


References
-
- Hallek M, Al-Sawaf O (2021) Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am J Hematol 96(12):1679–1705 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

