Pathological Complete Response in Patients With Resected Pancreatic Adenocarcinoma After Preoperative Chemotherapy
- PMID: 38888920
- PMCID: PMC11185983
- DOI: 10.1001/jamanetworkopen.2024.17625
Pathological Complete Response in Patients With Resected Pancreatic Adenocarcinoma After Preoperative Chemotherapy
Abstract
Importance: Preoperative chemo(radio)therapy is increasingly used in patients with localized pancreatic adenocarcinoma, leading to pathological complete response (pCR) in a small subset of patients. However, multicenter studies with in-depth data about pCR are lacking.
Objective: To investigate the incidence, outcome, and risk factors of pCR after preoperative chemo(radio)therapy.
Design, setting, and participants: This observational, international, multicenter cohort study assessed all consecutive patients with pathology-proven localized pancreatic adenocarcinoma who underwent resection after 2 or more cycles of chemotherapy (with or without radiotherapy) in 19 centers from 8 countries (January 1, 2010, to December 31, 2018). Data collection was performed from February 1, 2020, to April 30, 2022, and analyses from January 1, 2022, to December 31, 2023. Median follow-up was 19 months.
Exposures: Preoperative chemotherapy (with or without radiotherapy) followed by resection.
Main outcomes and measures: The incidence of pCR (defined as absence of vital tumor cells in the sampled pancreas specimen after resection), its association with OS from surgery, and factors associated with pCR. Factors associated with overall survival (OS) and pCR were investigated with Cox proportional hazards and logistic regression models, respectively.
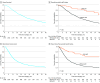
Results: Overall, 1758 patients (mean [SD] age, 64 [9] years; 879 [50.0%] male) were studied. The rate of pCR was 4.8% (n = 85), and pCR was associated with OS (hazard ratio, 0.46; 95% CI, 0.26-0.83). The 1-, 3-, and 5-year OS rates were 95%, 82%, and 63% in patients with pCR vs 80%, 46%, and 30% in patients without pCR, respectively (P < .001). Factors associated with pCR included preoperative multiagent chemotherapy other than (m)FOLFIRINOX ([modified] leucovorin calcium [folinic acid], fluorouracil, irinotecan hydrochloride, and oxaliplatin) (odds ratio [OR], 0.48; 95% CI, 0.26-0.87), preoperative conventional radiotherapy (OR, 2.03; 95% CI, 1.00-4.10), preoperative stereotactic body radiotherapy (OR, 8.91; 95% CI, 4.17-19.05), radiologic response (OR, 13.00; 95% CI, 7.02-24.08), and normal(ized) serum carbohydrate antigen 19-9 after preoperative therapy (OR, 3.76; 95% CI, 1.79-7.89).
Conclusions and relevance: This international, retrospective cohort study found that pCR occurred in 4.8% of patients with resected localized pancreatic adenocarcinoma after preoperative chemo(radio)therapy. Although pCR does not reflect cure, it is associated with improved OS, with a doubled 5-year OS of 63% compared with 30% in patients without pCR. Factors associated with pCR related to preoperative chemo(radio)therapy regimens and anatomical and biological disease response features may have implications for treatment strategies that require validation in prospective studies because they may not universally apply to all patients with pancreatic adenocarcinoma.
Conflict of interest statement
Figures
Similar articles
-
Adjuvant Chemotherapy After Resection of Localized Pancreatic Adenocarcinoma Following Preoperative FOLFIRINOX.JAMA Oncol. 2025 Mar 1;11(3):276-287. doi: 10.1001/jamaoncol.2024.5917. JAMA Oncol. 2025. PMID: 39847363
-
Prognosis Associated with Complete Pathological Response Following Neoadjuvant Treatment for PancreaTic AdenOcarciNOma in the FOFLIRINOX Era: the Multicenter TONO Study.Ann Surg Oncol. 2025 Apr;32(4):2809-2818. doi: 10.1245/s10434-024-16735-2. Epub 2025 Jan 8. Ann Surg Oncol. 2025. PMID: 39777595
-
Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment.JAMA Oncol. 2020 Nov 1;6(11):1733-1740. doi: 10.1001/jamaoncol.2020.3537. JAMA Oncol. 2020. PMID: 32910170 Free PMC article.
-
Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial.Lancet Gastroenterol Hepatol. 2021 Feb;6(2):128-138. doi: 10.1016/S2468-1253(20)30330-7. Epub 2020 Dec 16. Lancet Gastroenterol Hepatol. 2021. PMID: 33338442 Clinical Trial.
-
Response and Survival Associated With First-line FOLFIRINOX vs Gemcitabine and nab-Paclitaxel Chemotherapy for Localized Pancreatic Ductal Adenocarcinoma.JAMA Surg. 2020 Sep 1;155(9):832-839. doi: 10.1001/jamasurg.2020.2286. JAMA Surg. 2020. PMID: 32667641 Free PMC article.
Cited by
-
Evolving survival patterns in pancreatic adenocarcinoma: a 23-year retrospective observational analysis.J Gastrointest Oncol. 2025 Jun 30;16(3):1280-1286. doi: 10.21037/jgo-2024-942. Epub 2025 Jun 26. J Gastrointest Oncol. 2025. PMID: 40672068 Free PMC article.
-
Impact of Surgical Resection After Induction Gemcitabine Plus S-1-Based Chemoradiotherapy in Patients with Locally Advanced Pancreatic Ductal Adenocarcinoma: A Focus on UR-LA Cases.Cancers (Basel). 2025 Mar 20;17(6):1048. doi: 10.3390/cancers17061048. Cancers (Basel). 2025. PMID: 40149381 Free PMC article.
-
Advances in neoadjuvant therapy for pancreatic cancer: Current trends and future directions.World J Clin Oncol. 2025 Jun 24;16(6):105849. doi: 10.5306/wjco.v16.i6.105849. World J Clin Oncol. 2025. PMID: 40585822 Free PMC article. Review.
-
Adjuvant Chemotherapy After Resection of Localized Pancreatic Adenocarcinoma Following Preoperative FOLFIRINOX.JAMA Oncol. 2025 Mar 1;11(3):276-287. doi: 10.1001/jamaoncol.2024.5917. JAMA Oncol. 2025. PMID: 39847363
-
Validation of the PANAMA Score for Survival and Benefit of Adjuvant Therapy in Patients With Resected Pancreatic Cancer after Neoadjuvant FOLFIRINOX.Ann Surg. 2025 May 1;281(5):852-860. doi: 10.1097/SLA.0000000000006650. Epub 2025 Jan 31. Ann Surg. 2025. PMID: 39886770 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous