Web-Based Mindfulness-Based Cognitive Therapy for Adults With a History of Depression: Protocol for a Randomized Controlled Trial
- PMID: 38888958
- PMCID: PMC11220437
- DOI: 10.2196/53966
Web-Based Mindfulness-Based Cognitive Therapy for Adults With a History of Depression: Protocol for a Randomized Controlled Trial
Abstract
Background: Depression poses a major threat to public health with an increasing prevalence in the United States. Mindfulness-based interventions, such as mindfulness-based cognitive therapy (MBCT), are effective methods for managing depression symptoms and may help fortify existing efforts to address the current disease burden. The in-person group format of MBCT, however, incurs barriers to care such as expenses, childcare needs, and transportation issues. Alternate delivery modalities such as MBCT delivered via the web can be investigated for their capacity to overcome these barriers and still reduce symptoms of depression with adequate feasibility and efficacy.
Objective: This study protocol aims to examine the feasibility and efficacy of MBCT delivered via the web for the treatment of depression.
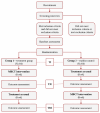
Methods: To attain study aims, 2 phases will be implemented using a waitlist control design. A total of 128 eligible participants will be randomized into either an 8-week MBCT intervention group plus treatment as usual (MBCT + TAU; group 1) or an 8-week waitlist control group (group 2). In phase I (8 weeks), group 1 will complete the intervention and group 2 will proceed with TAU. In phase II (8 weeks), group 2 will complete the intervention and group 1 will continue with TAU until reaching an 8-week follow-up. TAU may consist of receiving psychotherapy, pharmacotherapy, or combined treatment. Data collection will be completed at baseline, 8 weeks (postintervention for group 1 and preintervention for group 2), and 16 weeks (follow-up for group 1, postintervention for group 2). The primary outcomes will include (1) current, residual, or chronic depression symptoms and (2) psychiatric distress. Secondary outcomes will include perceived stress and facets of mindfulness. The feasibility will be measured by assessing protocol adherence, retention, attendance, and engagement. Finally, the extent of mindfulness self-practice and executive functioning skills will be assessed as mediators of intervention outcomes.
Results: This study began screening and recruitment in December 2022. Data collection from the first cohort occurred in January 2023. By November 2023, a total of 30 participants were enrolled out of 224 who received screening. Data analysis began in February 2024, with an approximate publication of results by August 2024. Institutional review board approval took place on September 11, 2019.
Conclusions: This trial will contribute to examining mindfulness-based interventions, delivered via the web, for improving current, residual, or chronic depression symptoms. It will (1) address the feasibility of MBCT delivered via the web; (2) contribute evidence regarding MBCT's efficacy in reducing depression symptoms and psychiatric distress; and (3) assess the impact of MBCT on several important secondary outcomes. Findings from this study will develop the understanding of the causal pathways between MBCT delivered via the web and depression symptoms further, elucidating the potential for future larger-scale designs.
Trial registration: ClinicalTrials.gov NCT05347719; https://www.clinicaltrials.gov/ct2/show/NCT05347719.
International registered report identifier (irrid): DERR1-10.2196/53966.
Keywords: MBCT; RCT; cognitive therapy; controlled trial; controlled trials; depression; depressive; depressive symptoms; distress; mental health; mindfulness; mindfulness-based cognitive therapy; mindfulness-based interventions; psychotherapy; randomized; remote; stress; virtual delivery.
©Mohammad Hooshmand Zaferanieh, Lu Shi, Meenu Jindal, Liwei Chen, Lingling Zhang, Snehal Lopes, Karyn Jones, Yucheng Wang, Kinsey Meggett, Cari Beth Walker, Grace Falgoust, Heidi Zinzow. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 18.06.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
Low-Intensity Guided Help Through Mindfulness (LIGHTMIND): study protocol for a randomised controlled trial comparing supported mindfulness-based cognitive therapy self-help to supported cognitive behavioural therapy self-help for adults experiencing depression.Trials. 2020 May 4;21(1):374. doi: 10.1186/s13063-020-04322-1. Trials. 2020. PMID: 32366320 Free PMC article.
-
Layperson-Facilitated Internet-Delivered Cognitive Behavioral Therapy for Homebound Older Adults With Depression: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2023 Feb 22;12:e44210. doi: 10.2196/44210. JMIR Res Protoc. 2023. PMID: 36811937 Free PMC article.
-
Study protocol of a multicenter randomized controlled trial comparing the effectiveness of group and individual internet-based Mindfulness-Based Cognitive Therapy with treatment as usual in reducing psychological distress in cancer patients: the BeMind study.BMC Psychol. 2015 Aug 13;3(1):27. doi: 10.1186/s40359-015-0084-1. eCollection 2015. BMC Psychol. 2015. PMID: 26273472 Free PMC article. Clinical Trial.
-
Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy with Older Adults: A Qualitative Review of Randomized Controlled Outcome Research.Clin Gerontol. 2019 Jul-Sep;42(4):347-358. doi: 10.1080/07317115.2018.1518282. Epub 2018 Sep 11. Clin Gerontol. 2019. PMID: 30204557 Review.
-
Mindfulness-based cognitive therapy for residual depressive symptoms and relapse prophylaxis.Curr Opin Psychiatry. 2016 Jan;29(1):7-12. doi: 10.1097/YCO.0000000000000216. Curr Opin Psychiatry. 2016. PMID: 26575299 Free PMC article. Review.
Cited by
-
Next-Generation Cognitive-Behavioral Therapy for Depression: Integrating Digital Tools, Teletherapy, and Personalization for Enhanced Mental Health Outcomes.Medicina (Kaunas). 2025 Feb 28;61(3):431. doi: 10.3390/medicina61030431. Medicina (Kaunas). 2025. PMID: 40142242 Free PMC article.
References
-
- World Health Organization The global burden of disease: 2004 update. World Health Organization. 2008. [2023-06-19]. https://apps.who.int/iris/handle/10665/43942 .
-
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/208671 62/6/617 - DOI - PMC - PubMed
-
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0... 06-PLME-RA-0071R2 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical