Inhibition of the eukaryotic initiation factor-2α kinase PERK decreases risk of autoimmune diabetes in mice
- PMID: 38889047
- PMCID: PMC11324307
- DOI: 10.1172/JCI176136
Inhibition of the eukaryotic initiation factor-2α kinase PERK decreases risk of autoimmune diabetes in mice
Abstract
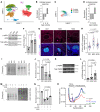
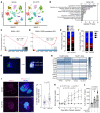
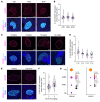
Preventing the onset of autoimmune type 1 diabetes (T1D) is feasible through pharmacological interventions that target molecular stress-responsive mechanisms. Cellular stresses, such as nutrient deficiency, viral infection, or unfolded proteins, trigger the integrated stress response (ISR), which curtails protein synthesis by phosphorylating eukaryotic translation initiation factor-2α (eIF2α). In T1D, maladaptive unfolded protein response (UPR) in insulin-producing β cells renders these cells susceptible to autoimmunity. We found that inhibition of the eIF2α kinase PKR-like ER kinase (PERK), a common component of the UPR and ISR, reversed the mRNA translation block in stressed human islets and delayed the onset of diabetes, reduced islet inflammation, and preserved β cell mass in T1D-susceptible mice. Single-cell RNA-Seq of islets from PERK-inhibited mice showed reductions in the UPR and PERK signaling pathways and alterations in antigen-processing and presentation pathways in β cells. Spatial proteomics of islets from these mice showed an increase in the immune checkpoint protein programmed death-ligand 1 (PD-L1) in β cells. Golgi membrane protein 1, whose levels increased following PERK inhibition in human islets and EndoC-βH1 human β cells, interacted with and stabilized PD-L1. Collectively, our studies show that PERK activity enhances β cell immunogenicity and that inhibition of PERK may offer a strategy for preventing or delaying the development of T1D.
Keywords: Beta cells; Diabetes; Endocrinology; Pharmacology.
Conflict of interest statement
Figures


Update of
-
Inhibition of the Eukaryotic Initiation Factor-2-α Kinase PERK Decreases Risk of Autoimmune Diabetes in Mice.bioRxiv [Preprint]. 2024 Jun 3:2023.10.06.561126. doi: 10.1101/2023.10.06.561126. bioRxiv. 2024. Update in: J Clin Invest. 2024 Jun 18;134(16):e176136. doi: 10.1172/JCI176136. PMID: 38895427 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

