Humoral Immunogenicity of mRNA-1345 RSV Vaccine in Older Adults
- PMID: 38889247
- PMCID: PMC11566230
- DOI: 10.1093/infdis/jiae316
Humoral Immunogenicity of mRNA-1345 RSV Vaccine in Older Adults
Abstract
Background: The mRNA-1345 vaccine demonstrated efficacy against respiratory syncytial virus (RSV) disease with acceptable safety in adults aged ≥60 years in the ConquerRSV trial. Here, humoral immunogenicity results from the trial are presented.
Methods: This phase 2/3 trial randomly assigned adults (≥60 years) to mRNA-1345 50-µg encoding prefusion F (preF) glycoprotein (n = 17 793) vaccine or placebo (n = 17 748). RSV-A and RSV-B neutralizing antibody (nAb) and preF binding antibody (bAb) levels at baseline and day 29 postvaccination were assessed in a per-protocol immunogenicity subset (PPIS; mRNA-1345, n = 1515; placebo, n = 333).
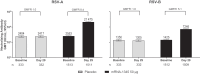
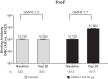
Results: Day 29 nAb geometric mean titers (GMTs) increased 8.4-fold against RSV-A and 5.1-fold against RSV-B from baseline. Seroresponses (4-fold rise from baseline) in the mRNA-1345 groups were 74.2% and 56.5% for RSV-A and RSV-B, respectively. Baseline GMTs were lower among participants who met the seroresponse criteria than those who did not. mRNA-1345 induced preF bAbs at day 29, with a pattern similar to nAbs. Day 29 antibody responses across demographic and risk subgroups were generally consistent with the overall PPIS.
Conclusions: mRNA-1345 enhanced RSV-A and RSV-B nAbs and preF bAbs in adults (≥60 years) across various subgroups, including those at risk for severe disease, consistent with its demonstrated efficacy in the prevention of RSV disease.
Clinical trials registration: NCT05127434.
Keywords: RSV; binding antibody; immunogenicity; mRNA-1345; neutralizing antibody.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. G. P. M. reports funding from Moderna, Merck, Sanofi, Pfizer, Universidad Nacional de San Martín, Medicago, GSK, and Cassara. C. J. A. D. reports receiving grants from the Medical Research Council and Wellcome, a consultancy for Synarigen, and is a member of the data and safety monitoring board at Oxford University. J. G., L. L., J. D., A. K., S. M., W. H., H. Z., S. K. S., F. P., N. L., C. A. S., E. W., J. M. M., and R. D. are employees of Moderna, Inc., and may hold stock/stock options in the company. N. L. C., K. S., and J. E. T. are consultants for Moderna, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Centers for Disease Control and Prevention . RSV in older adults and adults with chronic medical conditions. 2024. https://www.cdc.gov/rsv/older-adults/?CDC_AAref_Val=https://www.cdc.gov/rsv/high-risk/older-adults.html. Accessed 6 November 2023.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

